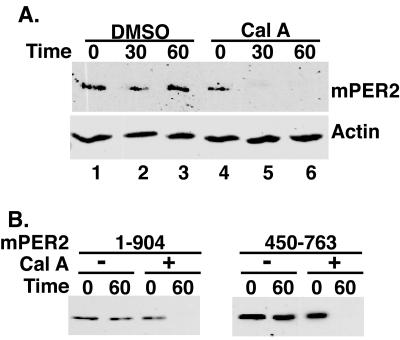
FIG. 1.
mPER2 stability is regulated by protein phosphorylation. (A) Phosphatase inhibition leads to rapid loss of full-length mPER2 protein. HEK293 cells transiently expressing Myc epitope-tagged full-length mPER2 protein were treated with cycloheximide (25 μg/ml) and either vehicle (lanes 1 to 3) or calyculin A (80 nM, lanes 4 to 6) for the length of time indicated. The abundance of mPER2 was assessed by Western blotting of lysates with 9E10 anti-Myc monoclonal antibodies. As a loading control, the blot was stripped and reprobed with anti-actin polyclonal antibodies. (B) Definition of a minimal domain required for phosphatase-induced destabilization of mPER2. The indicated fragments of mPER2 were expressed, treated, and analyzed as for panel A. Cal A, calyculin A.