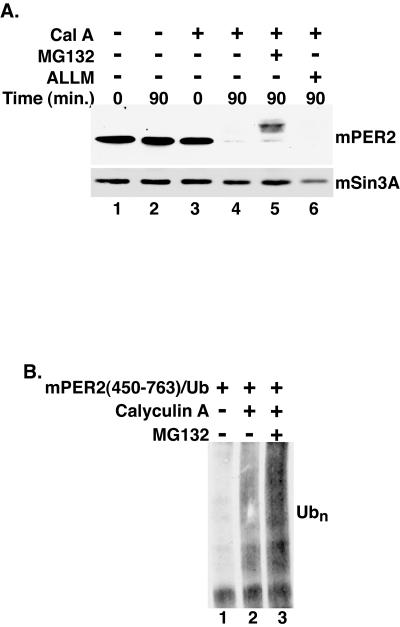
FIG. 2.
Phosphorylated mPER2 is degraded by the 26S proteasome. (A) Proteasome inhibition delays mPER2 degradation. Myc epitope-tagged mPER2(450-763) was transiently expressed in HEK293 cells. Five hours after the addition of the proteasome inhibitor MG132 (20 μM) (lane 5) or the calpain inhibitor ALLM (50 μM) (lane 6) to the growth medium, cells were treated with vehicle (lanes 1 and 2) or with calyculin A (Cal A; 80 nM) (lanes 3 to 6) and harvested at the indicated times, as in Fig. 1. As a loading control, the blot was stripped and reprobed with polyclonal antibodies that recognize mSin3A (lower panel). (B) Phosphorylation stimulates the polyubiquitination (Ubn) of mPER2. Myc epitope-tagged mPER2(450-764) was coexpressed with HA epitope-tagged ubiquitin and treated with combinations of calyculin A (80 nM) and MG132 (20 μM) as indicated in the figure. mPER2(450-764) was immunoprecipitated with 9E10 anti-Myc monoclonal antibodies, and the degree of ubiquitination was assessed by Western blotting with anti-HA monoclonal antibodies.