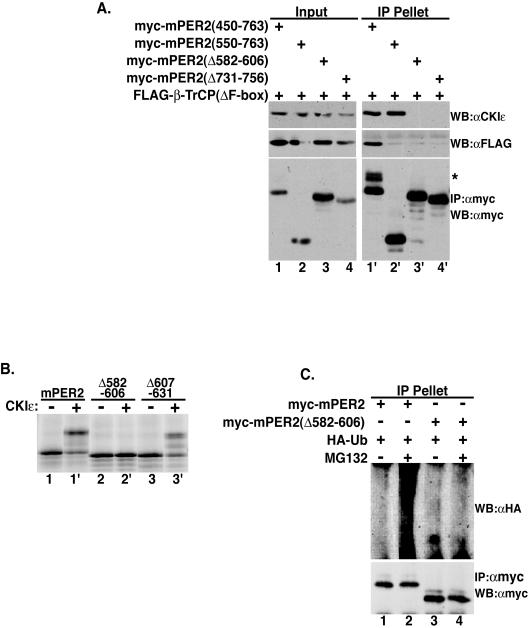
FIG. 7.
Stabilizing deletions in mPER2 block its interaction with β-TrCP(ΔF-box) and CKIɛ. (A) Deletions that block CKIɛ binding also block β-TrCP binding. Myc epitope-tagged mPER2(450-763) with or without the indicated internal deletions were coexpressed with FLAG epitope-tagged β-TrCP(ΔF-box) in HEK 293 cells. mPER2 was immunoprecipitated (IP) with 9E10 anti-Myc monoclonal antibodies, and the presence of β-TrCP(ΔF-box) or endogenous CKIɛ in the immunoprecipitation pellet was determined by Western blotting (WB) with anti-CKIɛ polyclonal antibodies (upper right panel) or anti-FLAG monoclonal antibodies (middle right panel). To confirm the presence of mPER2 in the immunoprecipitation pellet, the blot was stripped and reprobed with anti-Myc antibodies (lower right panel). The asterisk indicates mPER2 mobility shift due to binding of β-TrCP(ΔF-box), as described in the legend to Fig. 4A and B. (B) Deletion of the CKIɛ binding site prevents mPER2 phosphorylation. In vitro synthesized and [35S]methionine-labeled mPER2 or mPER2 with internal deletions was incubated with 50 ng of recombinant CKIɛ(Δ319) (+). Mobility shifts were analyzed after SDS-PAGE and PhosphorImager analysis. (C) Loss of CKIɛ binding sites prevents mPER2 polyubiquitination. Myc epitope-tagged mPER2(450-763) (lanes 1 and 2) or mPER2(450-764) (Δ582-606) (lanes 3 and 4) was coexpressed with HA epitope-tagged ubiquitin (HA-Ub). mPER2 was immunoprecipitated with 9E10 anti-Myc antibodies, and ubiquitination was determined by Western blotting with anti-HA antibodies.