Abstract
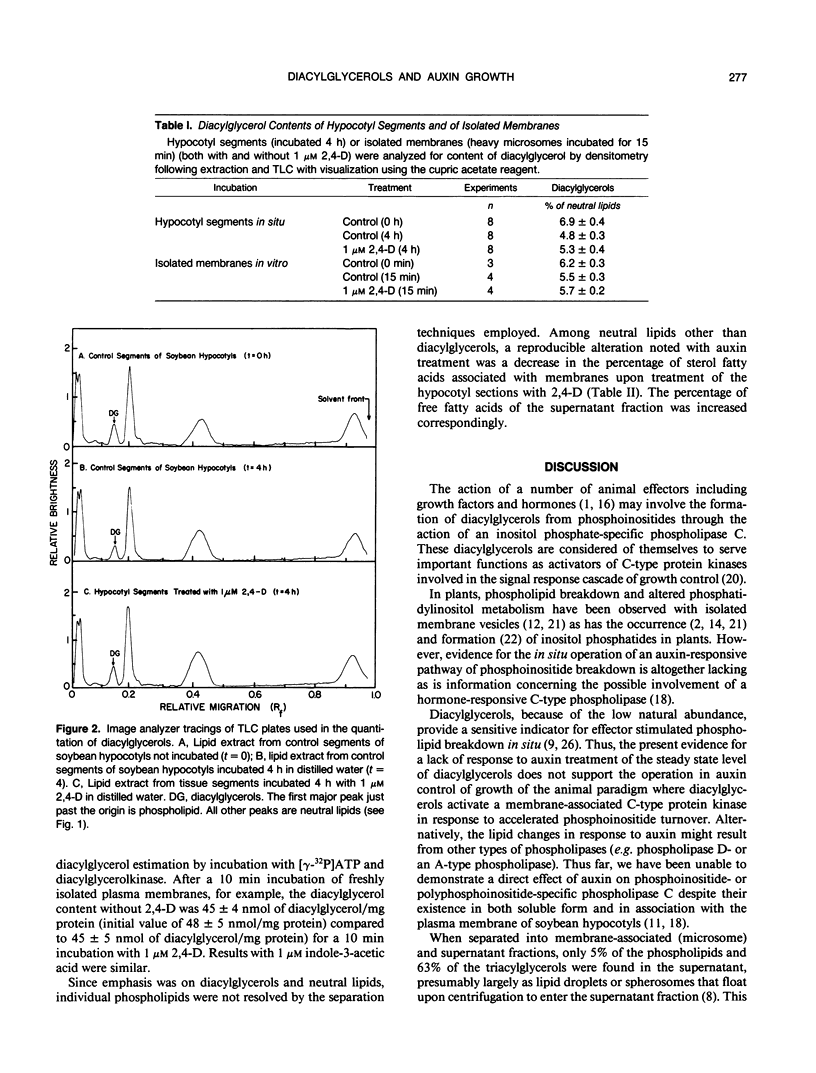
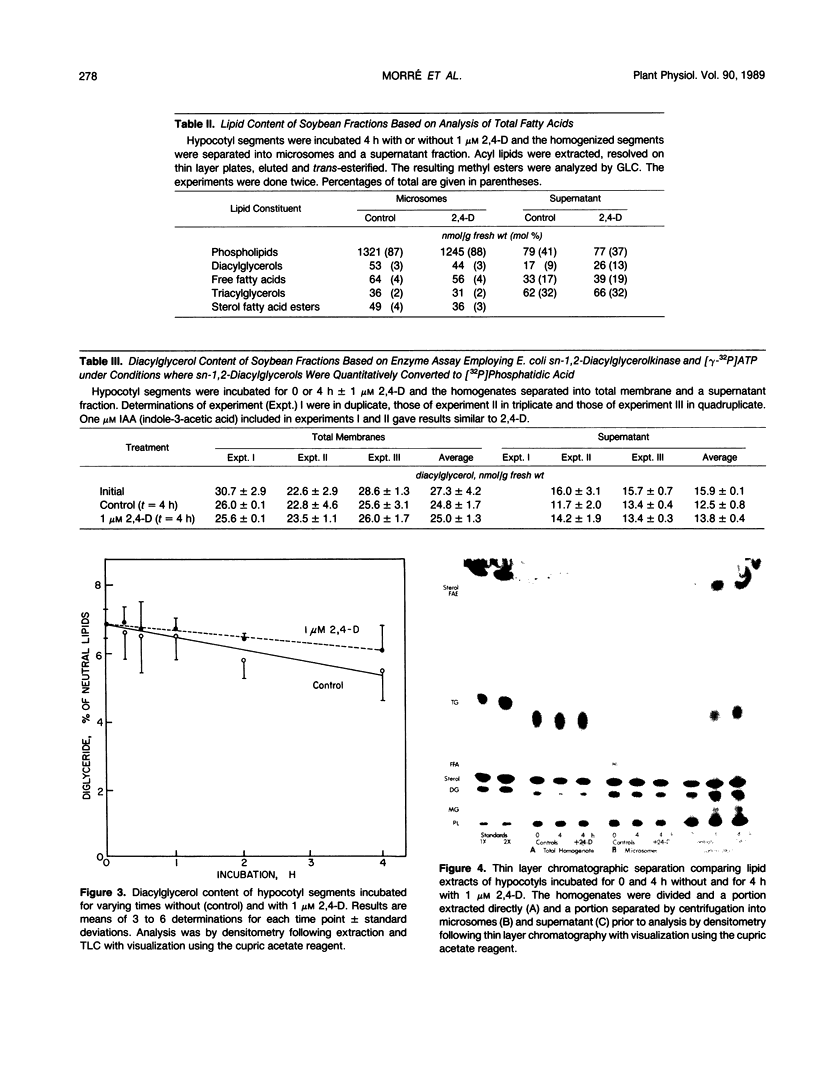
Diacylglycerol contents of excised soybean (Glycine max L.) hypocotyl segments, incubated for 4 hours in the presence or absence of a growth promoting concentration of 2,4-dichlorophenoxyacetic acid (2,4-D) were monitored by three different methods as a sensitive measure of the action in vivo of C-type phospholipases. By all three methods, steady state levels of diacylglycerols representing about 3% of the total lipids or about 7% of the neutral lipids, depending on method of assay, declined 18% over 4 hours of incubation as determined by extraction of total lipids and analysis by thin layer chromatography and densitometry. The average decline with 2,4-D-treated segments was less but the difference from controls was not significant. In those experiments where a small effect of 2,4-D was noted, the fraction showing an elevated diacylglycerol level in response to 2,4-D, after separation into membrane and supernatant fractions, was the supernatant and not the membranes. Results were confirmed from analyses of total fatty acids in each of the major lipid fractions and from diacylglycerol assays by conversion into phosphatidic acid upon incubation with [γ-32P]ATP and purified diacylglycerol phosphokinase from Escherichia coli. In the presence of 2,4-D, the diacylglycerol content of the membranes was unchanged compared to membranes from control segments. As with the densitometric method, the small 2,4-D induced increase in diacylglycerols, when observed, was insignificant and in the supernatant. The only membrane-associated lipid fraction consistently showing a response to 2,4-D was the fraction containing sterols esterified with fatty acids. Either total microsomes or purified plasma membranes when incubated for 10 to 20 minutes with 1 micromolar 2,4-D showed no accelerated formation of diacylglycerols compared to membranes not incubated. The results do not support operation during auxin growth of the animal paradigm where diacylglycerol activation of C-type protein kinases occurs in response to activated phospholipase C breakdown of phosphoinositides.
Full text
PDF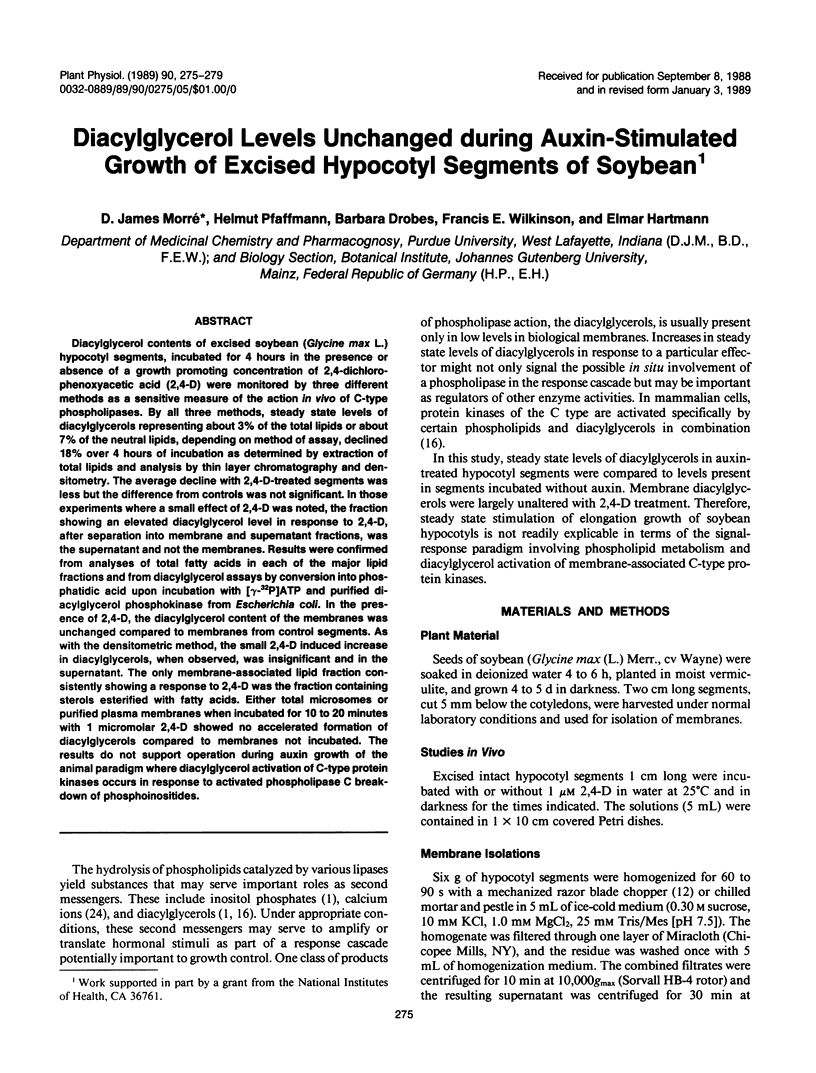


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Boss W. F., Massel M. O. Polyphosphoinositides are present in plant tissue culture cells. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1018–1023. doi: 10.1016/0006-291x(85)91908-4. [DOI] [PubMed] [Google Scholar]
- Donnelly T. E., Jr, Jensen R. Effect of fluphenazine on the stimulation of calcium-sensitive phospholipid-dependent protein kinase by 12-O-tetradecanoyl phorbol-13-acetate. Life Sci. 1983 Nov 28;33(22):2247–2253. doi: 10.1016/0024-3205(83)90298-9. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fewster M. E., Burns B. J., Mead J. F. Quantitative densitometric thin-layer chromatography of lipids using copper acetate reagent. J Chromatogr. 1969 Aug 5;43(1):120–126. doi: 10.1016/s0021-9673(00)99173-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacal J. C., de la Peña P., Moscat J., Garcia-Barreno P., Anderson P. S., Aaronson S. A. Rapid stimulation of diacylglycerol production in Xenopus oocytes by microinjection of H-ras p21. Science. 1987 Oct 23;238(4826):533–536. doi: 10.1126/science.2821623. [DOI] [PubMed] [Google Scholar]
- Melin P. M., Sommarin M., Sandelius A. S., Jergil B. Identification of Ca2+-stimulated polyphosphoinositide phospholipase C in isolated plant plasma membranes. FEBS Lett. 1987 Oct 19;223(1):87–91. doi: 10.1016/0014-5793(87)80515-x. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Gripshover B., Monroe A., Morré J. T. Phosphatidylinositol turnover in isolated soybean membranes stimulated by the synthetic growth hormone 2,4-dichlorophenoxyacetic acid. J Biol Chem. 1984 Dec 25;259(24):15364–15368. [PubMed] [Google Scholar]
- Morré D. J., Morré J. T., Varnold R. L. Phosphorylation of membrane-located proteins of soybean in vitro and response to auxin. Plant Physiol. 1984 May;75(1):265–268. doi: 10.1104/pp.75.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. J., Crain R. C., Satter R. L. Phosphatidylinositol Cycle Metabolites in Samanea saman Pulvini. Plant Physiol. 1987 Mar;83(3):640–644. doi: 10.1104/pp.83.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- O'Brian C. A., Arthur W. L., Weinstein I. B. The activation of protein kinase C by the polyphosphoinositides phosphatidylinositol 4,5-diphosphate and phosphatidylinositol 4-monophosphate. FEBS Lett. 1987 Apr 20;214(2):339–342. doi: 10.1016/0014-5793(87)80083-2. [DOI] [PubMed] [Google Scholar]
- Pfaffmann H., Hartmann E., Brightman A. O., Morré D. J. Phosphatidylinositol specific phospholipase C of plant stems : membrane associated activity concentrated in plasma membranes. Plant Physiol. 1987 Dec;85(4):1151–1155. doi: 10.1104/pp.85.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Sandelius A. S., Morré D. J. Characteristics of a phosphatidylinositol exchange activity of soybean microsomes. Plant Physiol. 1987 Aug;84(4):1022–1027. doi: 10.1104/pp.84.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J Biol Chem. 1987 Mar 25;262(9):3944–3946. [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]