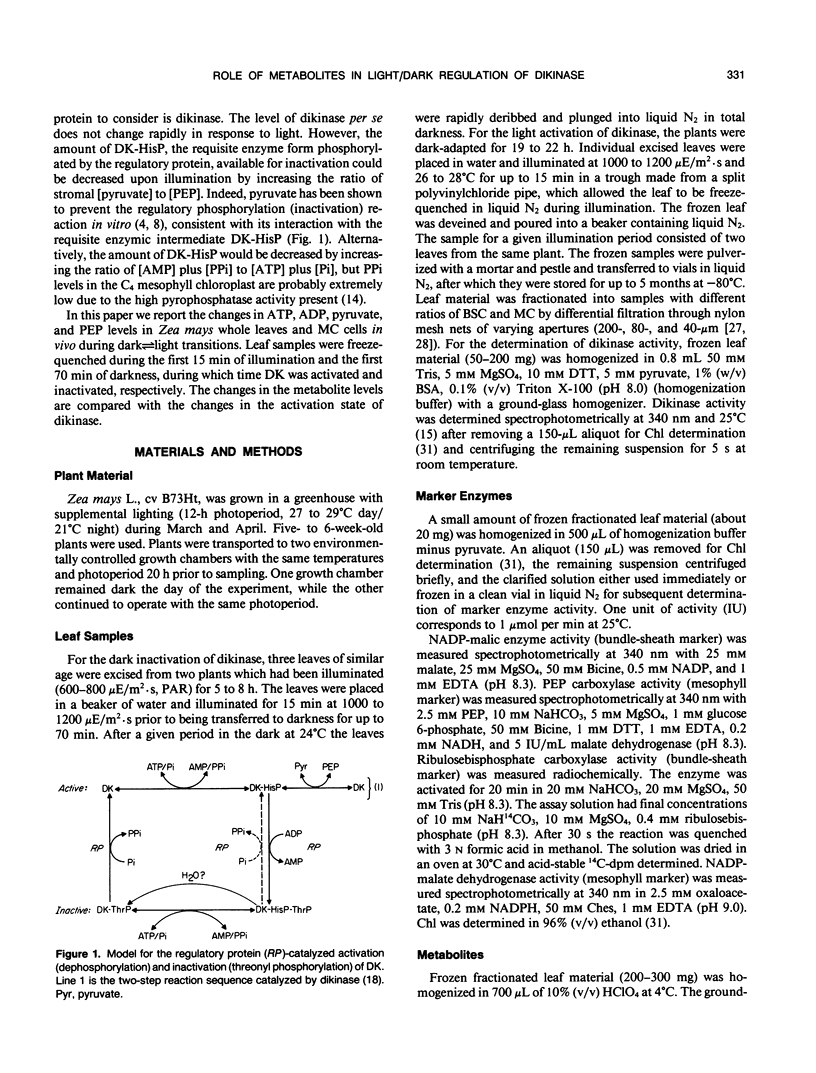
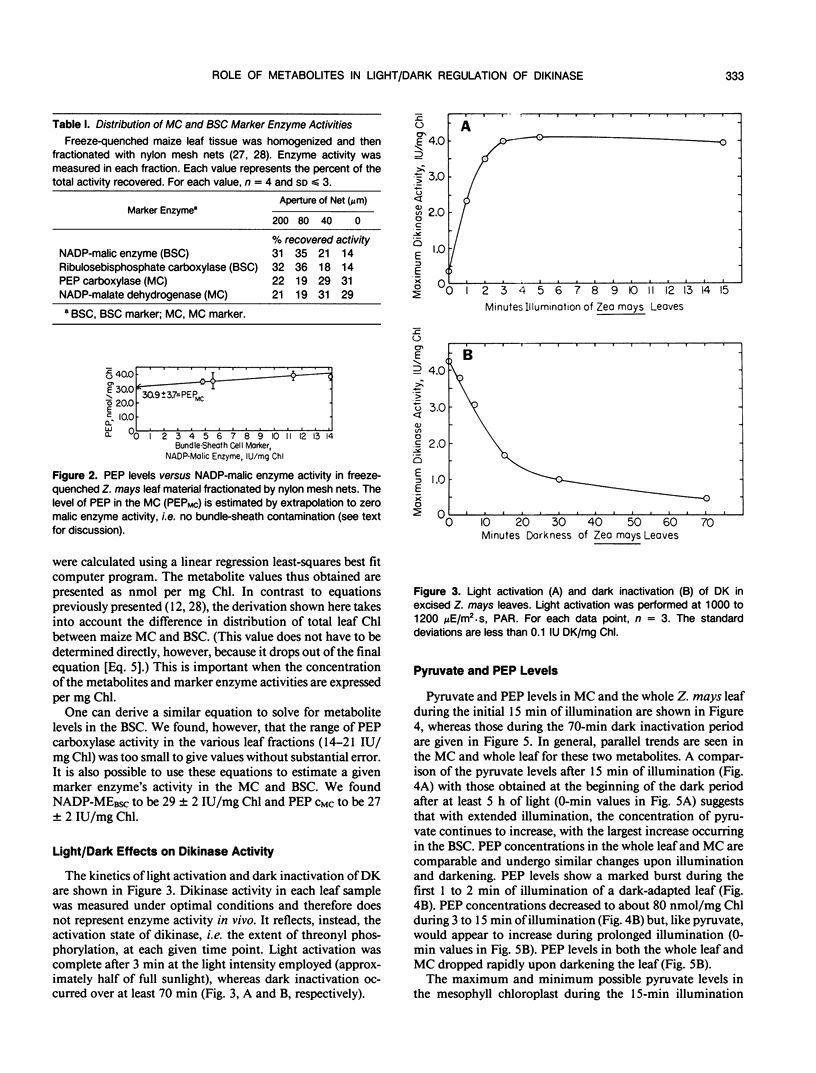
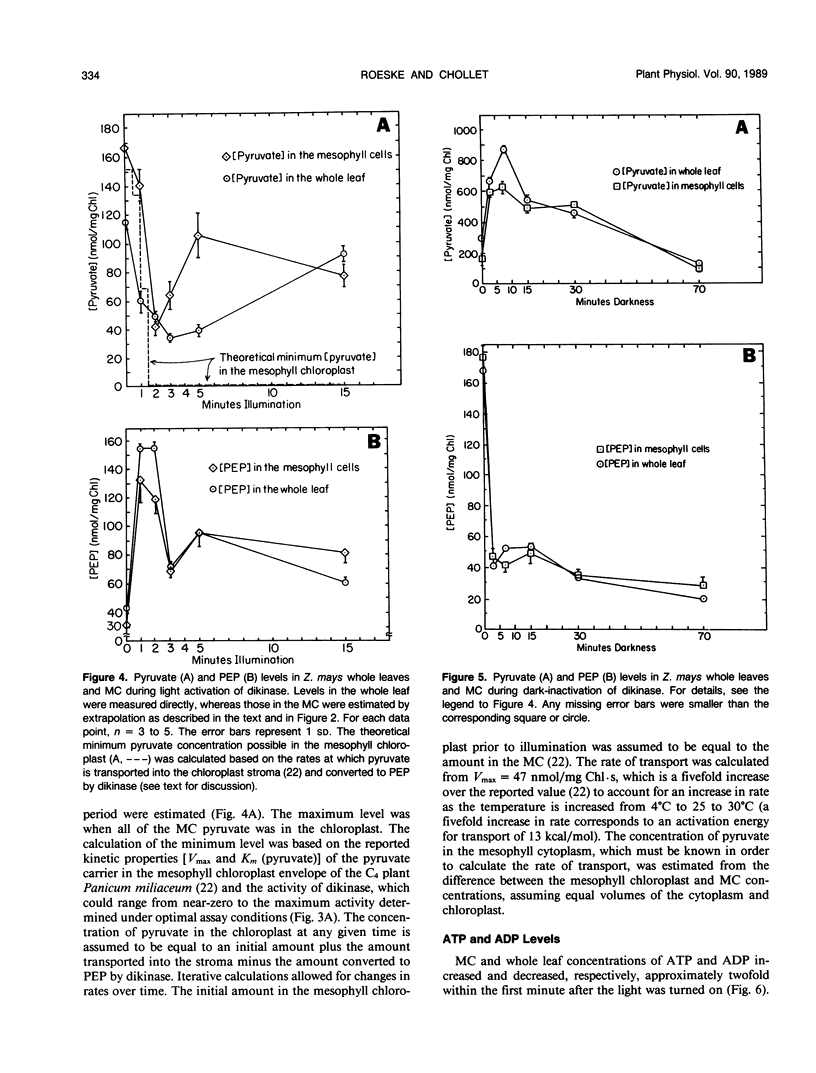
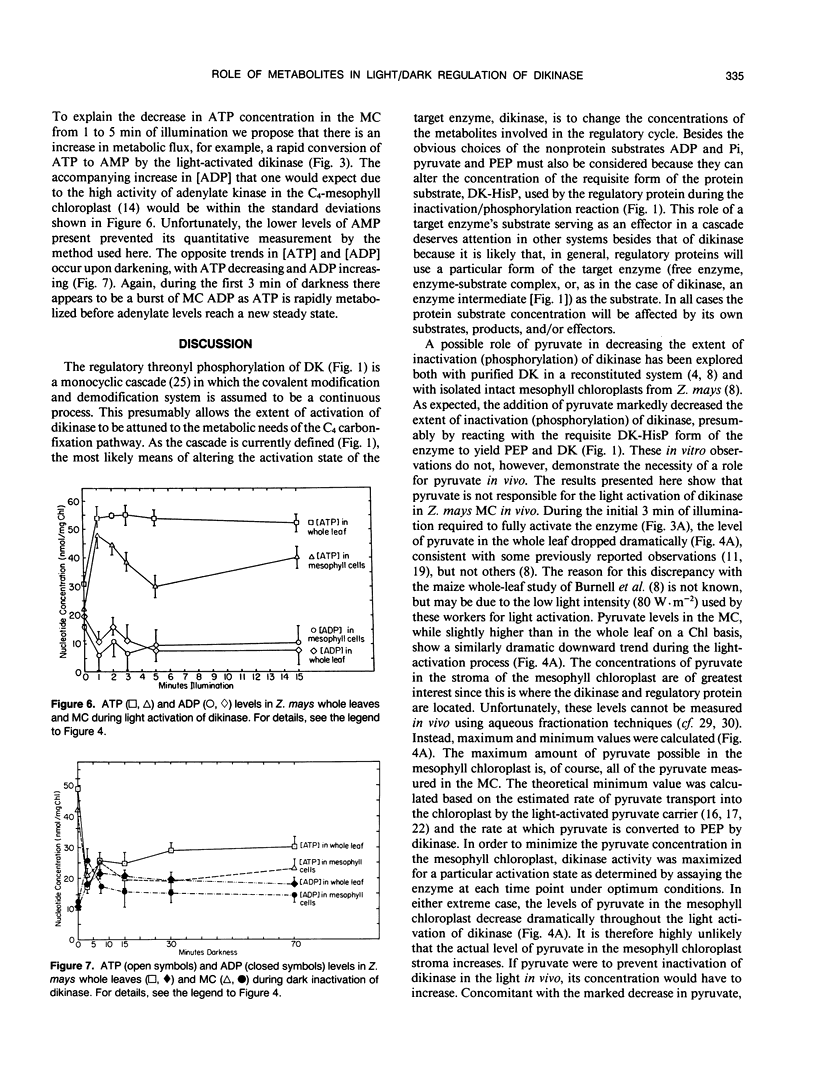
Abstract
Whole leaf and mesophyll cell concentrations of pyruvate, phosphoenolpyruvate (PEP), ATP, and ADP were determined in Zea mays during the reversible light activation of pyruvate, orthophosphate dikinase in vivo. Mesophyll cell levels of the four metabolites were estimated by extrapolation from values in freeze-quenched leaf samples that were fractionated by differential filtration through nylon mesh nets (adapted from M Stitt, HW Heldt [1985] Planta 164: 179-188). During the 3 minutes required for complete light activation of dikinase, pyruvate levels in the mesophyll cell decreased (from 166 ± 15 to 64 ± 10 nanomoles per milligram of chlorophyll [nmol/mg Chl]) while PEP levels increased (from 31 ± 4 to 68 ± 4 nmol/mg Chl, with a transient burst of 133 ± 16 nmol/mg Chl at 1 minute). Mesophyll cell levels of ATP increased (from 22 ± 4 to 48 ± 3 nmol/mg Chl) and ADP levels decreased (from 16 ± 4 to 7 ± 6 nmol/mg Chl) during the first minute of illumination. Upon darkening of the leaf and inactivation of dikinase, pyruvate levels initially increased in the mesophyll (from 160 ± 30 to a maximum of 625 ± 40 nmol/mg Chl), and then slowly decreased to about the initial value in the light over an hour. PEP levels dropped (from 176 ± 5 to 47 ± 3 nmol/mg Chl) in the first 3 minutes and remained low for the remainder of the dark period. Mesophyll levels of ATP and ADP rapidly decreased and increased, respectively, about twofold upon darkening. The trends observed for these metabolite levels in the mesophyll cell during the light/dark regulation of pyruvate, orthophosphate dikinase activity suggest that pyruvate and PEP do not play a major role in vivo in regulating the extent of light activation (dephosphorylation) or dark inactivation (ADP-dependent threonyl phosphorylation) of dikinase by its bifunctional regulatory protein. While the changes in ADP levels appear qualitatively consistent with a regulatory role for this metabolite in the light activation and dark inactivation of dikinase, they are not of a sufficient magnitude to account completely for the tenfold change in enzyme activity observed in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton A. R., Hatch M. D. Regulation of C4 photosynthesis: regulation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1983 Aug 30;115(1):53–60. doi: 10.1016/0006-291x(83)90967-1. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E., Walton G. M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967 Jul 10;242(13):3239–3241. [PubMed] [Google Scholar]
- Budde R. J., Ernst S. M., Chollet R. Substrate specificity and regulation of the maize (Zea mays) leaf ADP: protein phosphotransferase catalysing phosphorylation/inactivation of pyruvate, orthophosphate dikinase. Biochem J. 1986 Jun 1;236(2):579–584. doi: 10.1042/bj2360579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde R. J., Holbrook G. P., Chollet R. Studies on the dark/light regulation of maize leaf pyruvate, orthophosphate dikinase by reversible phosphorylation. Arch Biochem Biophys. 1985 Oct;242(1):283–290. doi: 10.1016/0003-9861(85)90503-x. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Regulation of C4 photosynthesis: catalytic phosphorylation as a prerequisite for ADP-mediated inactivation of pyruvate,Pi dikinase. Biochem Biophys Res Commun. 1984 Jan 13;118(1):65–72. doi: 10.1016/0006-291x(84)91068-4. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Regulation of C4 photosynthesis: purification and properties of the protein catalyzing ADP-mediated inactivation and Pi-mediated activation of pyruvate,Pi dikinase. Arch Biochem Biophys. 1985 Mar;237(2):490–503. doi: 10.1016/0003-9861(85)90302-9. [DOI] [PubMed] [Google Scholar]
- Burnell J. N. Regulation of C4 photosynthesis catalytic dephosphorylation and Pi-mediated activation of pyruvate Pi dikinase. Biochem Biophys Res Commun. 1984 Apr 30;120(2):559–565. doi: 10.1016/0006-291x(84)91291-9. [DOI] [PubMed] [Google Scholar]
- Day D. A., Hatch M. D. Transport of 3-phosphoglyceric acid, phosphoenolpyruvate, and inorganic phosphate in maize mesophyll chloroplasts,, and the effect of 3-phosphoglyceric acid on malate and phosphoenolpyruvate production. Arch Biochem Biophys. 1981 Oct 15;211(2):743–749. doi: 10.1016/0003-9861(81)90511-7. [DOI] [PubMed] [Google Scholar]
- Foyer C., Walker D., Spencer C., Mann B. Observations on the phosphate status and intracellular pH of intact cells, protoplasts and chloroplasts from photosynthetic tissue using phosphorus-31 nuclear magnetic resonance. Biochem J. 1982 Feb 15;202(2):429–434. doi: 10.1042/bj2020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Heldt H. W. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984 Jul;75(3):542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Stitt M., Heldt H. W. Subcellular Metabolite Levels in Spinach Leaves : Regulation of Sucrose Synthesis during Diurnal Alterations in Photosynthetic Partitioning. Plant Physiol. 1987 Feb;83(2):399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Transport in C4 mesophyll chloroplasts characterization of the pyruvate carrier. Biochim Biophys Acta. 1977 Dec 23;462(3):583–602. doi: 10.1016/0005-2728(77)90103-7. [DOI] [PubMed] [Google Scholar]
- Jenkins C. L., Hatch M. D. Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase. Arch Biochem Biophys. 1985 May 15;239(1):53–62. doi: 10.1016/0003-9861(85)90811-2. [DOI] [PubMed] [Google Scholar]
- Nakamoto H., Edwards G. E. Control of the activation/inactivation of pyruvate, Pi dikinase from the C4 plant maize by adenylate energy charge, pyruvate, and analogs of pyruvate. Biochem Biophys Res Commun. 1983 Sep 15;115(2):673–679. doi: 10.1016/s0006-291x(83)80197-1. [DOI] [PubMed] [Google Scholar]
- Nakamoto H., Edwards G. E. Light Activation of Pyruvate,Pi Dikinase and NADP-Malate Dehydrogenase in Mesophyll Protoplasts of Maize : Effect of DCMU, Antimycin A, CCCP, and Phlorizin. Plant Physiol. 1986 Sep;82(1):312–315. doi: 10.1104/pp.82.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeske C. A., Chollet R. Chemical modification of the bifunctional regulatory protein of maize leaf pyruvate,orthophosphate dikinase. Evidence for two distinct active sites. J Biol Chem. 1987 Sep 15;262(26):12575–12582. [PubMed] [Google Scholar]
- Roeske C. A., Kutny R. M., Budde R. J., Chollet R. Sequence of the phosphothreonyl regulatory site peptide from inactive maize leaf pyruvate, orthophosphate dikinase. J Biol Chem. 1988 May 15;263(14):6683–6687. [PubMed] [Google Scholar]
- Stadtman E. R., Chock P. B. Superiority of interconvertible enzyme cascades in metabolic regulation: analysis of monocyclic systems. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2761–2765. doi: 10.1073/pnas.74.7.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H. Adenine Nucleotide Levels, the Redox State of the NADP System, and Assimilatory Force in Nonaqueously Purified Mesophyll Chloroplasts from Maize Leaves under Different Light Intensities. Plant Physiol. 1988 Dec;88(4):1461–1468. doi: 10.1104/pp.88.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H. Nonaqueous purification of maize mesophyll chloroplasts. Plant Physiol. 1988 Jun;87(2):427–430. doi: 10.1104/pp.87.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


