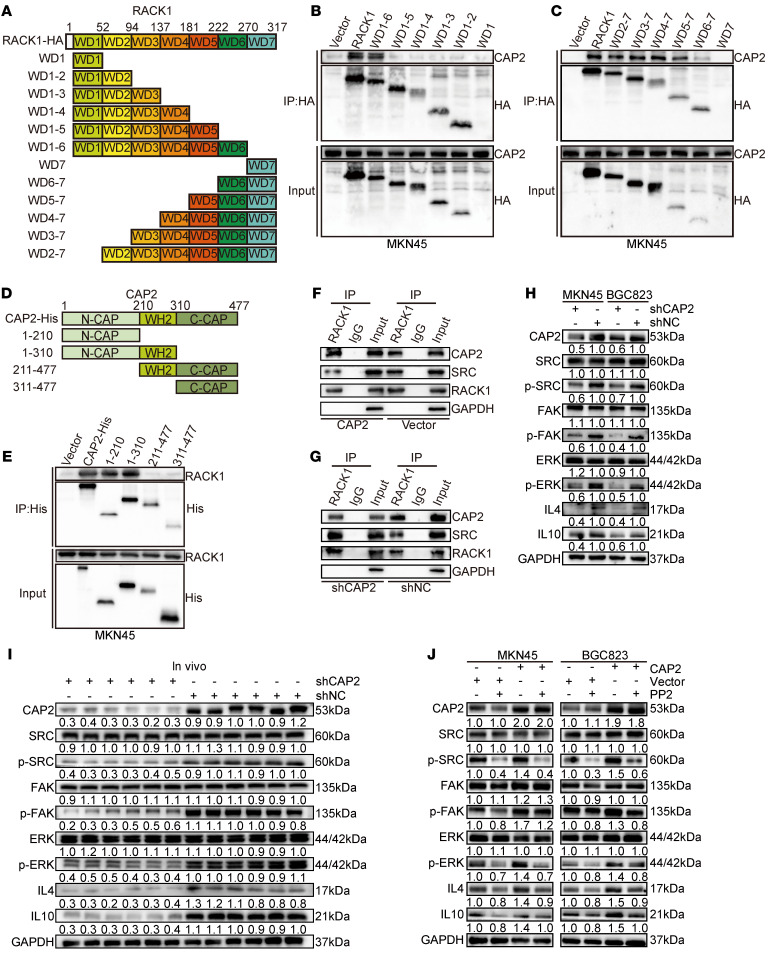
Figure 5. CAP2 competitively binds to domains WD5–WD7 of RACK1 and dissociates SRC.
(A) Schematic representation of the RACK1 domains. (B) HA-tagged WT-RACK1, WD6-1, WD5-1, WD4-1, WD3-1, WD2-1, and WD1 were individually overexpressed in MKN45 cells, and anti-HA–tagged antibodies were used for co-IP. (C) HA-tagged WT-RACK1, WD2-7, WD3-7, WD4-7, WD5-7, WD6-7, and WD7 were individually overexpressed in MKN45 cells, and anti-HA–tagged antibodies were used for co-IP. (D) Schematic diagram of CAP2 protein truncation. (E) Overexpression of His-tagged WT-CAP2, CAP2(1–210bp), CAP2(1–310bp), CAP2(211–477bp), and CAP2(311–477bp) in MKN45 cells, immunoprecipitated with an anti-His-tag antibody. (F and G) Immunoprecipitation was performed using an IP-grade anti-RACK1 antibody. The level of SRC binding to RACK1 in GC cells with or without CAP2 expression was detected by Western blotting. (H) The expression and phosphorylation levels of the SRC/FAK/ERK signaling pathway in GC cells with or without CAP2 knockdown were detected by Western blotting. (I) Western blotting was conducted to determine the expression and phosphorylation levels of SRC/FAK/ERK/IL-4 and IL-10 in xenografted tumors. (J) SRC inhibitor (PP2) was added to GC cells overexpressing CAP2, and the expression and phosphorylation levels of SRC/FAK/ERK/IL-4 and IL-10 were detected by Western blotting.