Abstract
Long-chain acyl-coenzyme A (CoA) synthase 4 (ACSL4) is an enzyme that esterifies CoA into specific polyunsaturated fatty acids, such as arachidonic acid and adrenic acid. Based on accumulated evidence, the ACSL4-catalyzed biosynthesis of arachidonoyl-CoA contributes to the execution of ferroptosis by triggering phospholipid peroxidation. Ferroptosis is a type of programmed cell death caused by iron-dependent peroxidation of lipids; ACSL4 and glutathione peroxidase 4 positively and negatively regulate ferroptosis, respectively. In addition, ACSL4 is an essential regulator of fatty acid (FA) metabolism. ACSL4 remodels the phospholipid composition of cell membranes, regulates steroidogenesis, and balances eicosanoid biosynthesis. In addition, ACSL4-mediated metabolic reprogramming and antitumor immunity have attracted much attention in cancer biology. Because it facilitates the cross-talk between ferroptosis and FA metabolism, ACSL4 is also a research hotspot in metabolic diseases and ischemia/reperfusion injuries. In this review, we focus on the structure, biological function, and unique role of ASCL4 in various human diseases. Finally, we propose that ACSL4 might be a potential therapeutic target.
Keywords: Long-chain acyl-coenzyme A (CoA) synthase 4 (ACSL4), Ferroptosis, Fatty acid metabolism, Cancer, Ischemia/reperfusion, Metabolic diseases
Introduction
Fatty acids (FAs) are composed of a hydrocarbon main chain and a terminal carboxylic acid group.[1] FAs are not only important fuel sources providing adenosine triphosphate (ATP) to cells through mitochondrial β-FA oxidation, but also structural components of triacylglycerols (TGs), phospholipids (PLs), cholesterol esters (CEs), and hormones.[2] The first step in the utilization of FAs by cells is activation by acyl-coenzyme A (acyl-CoA) synthases (ACS), which catalyze the conversion of free FAs to fatty acyl-CoAs.[3] According to the carbon chain length of their substrates, the ACS family is classified into very long-chain acyl-CoA synthase, long-chain acyl-CoA synthase (ACSL), medium-chain acyl-CoA synthase, and short-chain acyl-CoA synthase.[4] ACSL catalyzes FAs with 10 to 22 carbons and is composed of five isoenzymes, including ACSL1, ACSL3, ACSL4, ACSL5, and ACSL6.[5] Although they have different substrates and are localized to different tissues and subcellular compartments, these isoenzymes all play a vital role in the uptake and intracellular metabolism of FAs.[6,7]
Among the ACSL family members, ACSL4 preferentially catalyzes the formation of arachidonoyl-CoA (AA-CoA) by inserting coenzyme A (CoA) into arachidonic acid (AA), also called 5,8,11,14-eicosatetraenoic acid (20:4n-6, ω-6).[8] Other polyunsaturated fatty acids (PUFAs), such as adrenic acid (AdA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), epoxyeicosatrienoic acids (EETs), and hydroxyeicosatetraenoic acids (HETEs), are also substrates of ACSL4.[9,10] Without enough glutathione peroxidase 4 (GPX4), AA-CoA is converted into lipid peroxides (LPOs) and accumulates on cellular membranes, triggering ferroptotic membrane ruptures,[11] indicating that ACSL4 is a vital initiator of ferroptosis. In addition, ACSL4 participates in FA metabolism by remodeling the PL composition and regulating the biosynthesis of eicosanoids and steroids.[12–14]
In this review, we summarize the updated achievements regarding the structure and biological function of ACSL4 and report how ACSL4-mediated ferroptosis and disordered lipid metabolism contribute to human diseases. Finally, we propose that ACLS4 might be an available therapeutic target for disease treatment.
ACSL4
Gene and structure
The gene ACSL4 was first isolated in 1998 by researchers searching for the genetic cause in two brothers diagnosed with Alport syndrome, elliptocytosis, and mental retardation.[15] Further research investigating another two families confirmed the specific association between ACSL4 and X-linked non-specific mental retardation.[16,17] Moreover, due to its intrinsic function as an acyl-CoA synthase, ACSL4 is involved in FA metabolism and is associated with intracellular fat content.[15,18–21] For example, the ACSL4 rs7887981 polymorphism was related to the concentration of fasting insulin and triglycerides and liver fat content in Swedish men,[22] suggesting that genetic changes in ACSL4 might lead to abnormal metabolism and metabolic diseases.
ACSL4 is located on chromosome Xq23, and differential splicing can generate two different isoforms.[23] ACSL4 variant 1, the more abundant transcript, is localized to the cytoplasm and the inner layer of the cell membrane and is expressed in various organs, such as the kidneys, lungs, and pancreas.[24] However, ACSL4 variant 2, which has an additional 41 amino acids (N-terminal hydrophobic region), is located at the endoplasmic reticulum and lipid droplets and appears restricted to neurons.[24] Both variants prefer AA, AdA, EPA, and DHA as their substrates with a similar relative affinity but different reaction rates.[10]
Regulation
With an increasing number of studies focusing on ACSL4, several molecules suppressing ACSL4 have been identified.[25–27] The integrin α6β4 decreases the expression of ACSL4 by activating Src and signal transducer and activator of transcription 3, evading ferroptosis triggered by extracellular matrix detachment. However, the lack of α6β4 could not induce ferroptosis as expected because of sustained GPX4 expression despite an elevated ACSL4 level.[25] Intracellular vesicular transport factor p115 can bind ACSL4 with a high affinity and degrade ACSL4, which is enhanced by treating cells with AA, leading to a drastic decline in cellular ACSL4.[26] In addition, zinc lipoprotein A20 interacts with ACSL4 directly and suppresses its expression; meanwhile, microRNA (miR)-17-92 could protect against ferroptosis by targeting A20/ACSL4.[27] Interestingly, AA could also downregulate ACSL4 by promoting its ubiquitination and proteasomal degradation,[28] implicating that bidirectional regulation might exist between ACSL4 and AA.
ACSL4 expression can be transcriptionally increased by some transcription factors (TFs). A sequence analysis revealed many binding sites for TFs at the promoter of ACSL4, such as specificity protein 1 (Sp1) and cyclic adenosine monophosphate (cAMP) response element-binding (CREB).[29] By functional characterization, the Sp1 binding site was shown to be involved in basal activity, and the CREB site is involved in cAMP stimulation of ACSL4 transcription.[29] Sp1 can induce the transcriptional expression of ACSL4 under both normoxic and hypoxic conditions, and inhibiting Sp1 attenuates cell injury and lipid peroxidation in a hypoxia/reoxygenation model.[30] The transcriptional enhanced associate domain 4 (TEAD4) could also upregulate ACSL4 by binding its promoter, which could be enhanced by its coactivator Yes-associated protein (YAP).[31] Notably, peroxisome proliferator-activated receptor delta (PPARδ) is a tissue-specific transcriptional activator of ACSL4 in hepatocytes. PPARδ activation by its ligands could induce elevated messenger RNA (mRNA) and protein levels of ACSL4, and the hepatic depletion of PPARδ suppresses ACSL4 expression.[32] Sentrin-specific protease 1, a human protease that deconjugates small ubiquitin-like modifier (SUMO) molecules from SUMOylated proteins, could mediate the deSUMOylation of ACSL4 under hypoxia.[33] Tyrosine phosphatase Src homology-2 domain-containing protein tyrosine phosphatase-2 (SHP2), which is activated in a cAMP-dependent manner, could increase the expression of ACSL4 and the subsequent production of AA-CoAs and steroids,[34] although the detailed mechanism needs to be further clarified. The effects of these molecules regulating ACSL4 expression are summarized in Table 1.
Table 1.
The signaling pathways and molecules regulating ACSL4 expression.
| Cell or animal models | Signaling pathways or molecules | ACSL4 expression | Effect | References |
| MCF10-A, Hs578T, and SUM-159 | Integrin α6β4-mediated activation of Src and STAT3 | Downregulate | Protecting against changes of membrane lipid compositions and ferroptosis | [25] |
| HepG2; C57BL/6 J mice with a HFD | p115 binding to and mediating degradation of ACSL4 | Downregulate | UN | [26] |
| HUVEC cells | A20 interacting with ACSL4 directly | Downregulate | Protecting against ferroptosis | [27] |
| HepG2 and Huh7; primary hepatocytes | AA-mediated ubiquitin-proteasomal degradation of ACSL4 | Downregulate | UN | [28] |
| Caco-2 with hypoxia/reoxygenation; C57BL/6 mice with I/R | Sp1 binding to the promoter region of ACSL4 | Upregulate | Promoting ferroptosis | [29,30] |
| Human epithelial tumor cells and mesothelioma cells | Activation of Merlin/Hippo/YAP/TEAD4 pathway | Upregulate | Promoting ferroptosis | [31] |
| HepG2 and AML12; primary hepatocytes; Syrian hamsters | Activation of PPARδ | Upregulate | UN | [32] |
| H9c2 under hypoxia | SENP1-mediated deSUMOylation of ACSL4 | – | Protecting against ferroptosis | [33] |
| MA-10 | cAMP-dependent pathway-mediated activation of SHP2 | Upregulate | Increasing the production of steroid | [34] |
| CRL-1619, CRL-3367, CRL-6475, and CRL-1642 | IFN-γ-mediated activation of JAK/STAT1/IRF1 | Upregulate | Increasing the esterification of AA into PLs; promoting ferroptosis | [111] |
AA: Arachidonic acid; ACSL4: Acyl-CoA synthase 4; AML12: Mouse liver cells; cAMP: Cyclic adenosine monophosphate; Caco-2: Colon carcinoma cells; CRL-1619, CRL-3367, and CRL-6475: Human and mouse melanoma cells; CRL-1642: Lewis lung cancer cells; H9c2: Rat cardiac myocytes; HepG2 and Huh7: Hepatoma cells; HFD: High-fat diet; Hs578T and SUM-159: Breast carcinoma cells; HUVEC: Human umbilical vein endothelial cells; IFN: Interferon; I/R: Ischemia/reperfusion; IRF1: Interferon regulatory factor 1; JAK: Janus kinase; MA-10: Leydig cells; MCF10-A: Breast epithelial cells; PLs: Phospholipids; PPARδ: Peroxisome proliferator-activated receptor delta; SENP1: Sentrin-specific protease 1; SHP2: Src homology-2 domain-containing protein tyrosine phosphatase-2; Sp1: Specificity protein 1; STAT: Signal transducer and activator of transcription; TEAD4: Transcriptional enhanced associate domain; UN: Unknown; YAP: Yes-associated protein; –: Not available.
Moreover, various miRs can downregulate ACSL4 by binding its 3′-UTR (untranslated region), such as miR-424-5p, miR-141-3p, miR-548p, miR-34a, miR-130a-3p, miR-211-5p, miR-205, miR-23a-3p, miR-3098-3p, miR-454-3p, miR-106b-5p, and miR-224-5p.[35–48] miR-347, miR-214-3p, and miR-142-3p promote ACSL4 expression.[49–51] We summarize the effect of these miRs when targeting ACSL4 in different cells or animal models in Table 2. In addition, researchers have reported several agents able to inhibit the activity of ACSL4, such as triacsin C, thiazolidinediones (TZDs), abemaciclib, PRGL493, and valnoctamide.[52–58]
Table 2.
Effect of miRs targeting ACSL4 in different cell or animal models.
| miRNAs | Cell or animal models | ACSL4 expression | Potential mechanism | Effect | References |
| miR-424-5p | Ovarian cancer cells | Downregulate | Binding to 3′-UTR of ACSL4 | Reducing erastin- and RSL3-induced ferroptosis | [35] |
| miR-141-3p | Osteoarthritis chondrocytes | Downregulate | Binding to 3′-UTR of ACSL4 | Regulating lipid metabolism and stimulating chondrocyte apoptosis | [36] |
| miR-548p | Huh-7 and HepG2; primary hepatocytes | Downregulate | Binding to 3′-UTR of ACSL4 | Decreasing cholesterol, FAs, and triglyceride synthesis | [37] |
| miR-34a | Porcine primary intramuscular preadipocytes | Downregulate | Binding to 3′-UTR of ACSL4 | Reducing lipid droplet formation during adipogenesis | [38] |
| miR-130a-3p | Neural stem cells from Sprague–Dawley rat embryos | Downregulate | Binding to 3′-UTR of ACSL4 | Promoting neuronal differentiation via regulating AKT/PI3K pathway | [39] |
| miR-211-5p | HCC tissues | Downregulate | Binding to 3′-UTR of ACSL4 | Impairing tumorigenesis and growth of HCC | [40] |
| miR-205 | HepG2 | Downregulate | Binding to 3′-UTR of ACSL4 | Suppression of miR-205, elevating cholesterol levels | [41] |
| miR-23a-3p | BALB/cAnN-nu mice; human HCC tissues | Downregulate | Binding to 3′-UTR of ACSL4 | Inhibition of miR-23a-3p induced ferroptosis in sorafenib-treated HCC cells | [42] |
| miR-3098-3p | OGD/R-induced HT22 | Downregulate | Binding to 3′-UTR of ACSL4 | Inhibiting ferroptosis and protecting cells from dysfunction | [43] |
| miR-454-3p | RCC cells | Downregulate | Binding to 3′-UTR of ACSL4 | Impeding proliferation, lipid accumulation, and oxidation | [44] |
| miR-106b-5p | Brain microvascular endothelial cells | Downregulate | Binding to 3′-UTR of ACSL4 | Inhibition of miR-106b-5p suppressed cell viability and enhanced ferroptosis | [45] |
| miR-224-5p | 3T3-L1 | Downregulate | Binding to 3′-UTR of ACSL4 | Increasing the concentration of free FAs at terminal differentiation of adipocytes | [46] |
| miR-670-3p | U87MG and A172 | Downregulate | – | Protecting against ferroptosis | [47] |
| miR-3595 | Baicalin-treated HSC-T6 | Downregulate | – | Exerting an anti-fibrotic effect | [48] |
| miR-347 | Cortical neurons | Upregulate | – | miR-347 overexpression induced neuronal apoptotic death | [49] |
| miR-214-3p | Cisplatin-treated TCMK-1 | Upregulate | – | miR-214-3p inhibitor alleviated ferroptosis | [50] |
| miR-142-3p | HepG2 with HBV infection | Upregulate | – | miR-142-3p inhibitor alleviated ferroptosis and inhibited hepatoma cell growth | [51] |
3T3-L1: Adipocytes; ACSL4: Acyl-CoA synthase 4; AKT: Protein kinase B; FA: Fatty acid; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; HSC–T6: Rat hepatic stellate cells; HT22: Hippocampal neuronal cell; Huh-7 and HepG2: Hepatoma cells; I/R: Ischemia/reperfusion; miR: MicroRNA; OGD/R: Oxygen and glucose deprivation/reoxygenation; PI3K: Phosphatidylinositol 3-kinase; RCC: Renal cell carcinoma; TCMK-1: Murine tubular epithelial cells; U87MG and A172: Human glioblastoma cells; UTR: Untranslated region; –: Not available.
Biological Function of ACSL4
The primary biological function of ACSL4 is to catalyze the formation of fatty acyl-CoA by inserting CoA into PUFAs, such as AA and AdA. These fatty acyl-CoAs either provide energy to cells through β-FA oxidation or enter the lipid biosynthesis pathway to produce AA-containing TGs, CEs, and PLs.[59] For example, to form AA-containing phosphatidylcholines (PCs) and phosphatidylethanolamines (PEs), lysophosphatidylcholine acyltransferase 3 (LPCAT3) catalyzes the incorporation of AA-CoA into lysophosphatidylcholine (LPC) and lysophosphatidylethanolamine (LPE).[60] Notably, excessive peroxidation of AA-containing PLs in the cellular membrane triggers ferroptosis in both normal and tumor cells, leading to extensive research investigating the essential role of ACSL4 in ferroptosis. In addition, ACSL4-catalyzed PUFA acyl-CoAs participate in the regulation of the PL composition, steroidogenesis, and eicosanoid biosynthesis.
ACSL4 and ferroptosis
A brief introduction to ferroptosis
Ferroptosis was first proposed by Dixon in 2012 when he searched for small-molecule compounds able to suppress RAS-mutant cancer cell growth.[61] Ferroptosis was then defined as a form of non-apoptotic programmed cell death characterized by an iron-dependent accumulation of lipid-based reactive oxygen species (ROS), especially polyunsaturated PL hydroperoxides.[61–63] The morphological changes of ferroptosis include a lost membrane integrity, swollen cytoplasm and organelles, and chromatin condensation.[64] Furthermore, the mitochondria of cells with ferroptosis exhibit a reduced volume, an increased membrane density, reduced or absent cristae, and rupture of the outer membrane.[61,64] Located at the intersection of the iron, lipid, glutathione (GSH), and mevalonate (MVA) metabolic pathways, ferroptosis is initiated and inhibited by a complex mechanism.[65]
Several studies have demonstrated that accumulated lipid peroxidation is a major signal that triggers ferroptosis.[66,67] The oxidative attack of the carbon–carbon double bonds of PUFA-containing PLs contributes to the rupture of cell membranes and, thereby, induces ferroptotic cell death.[68] There are two major methods for producing LPOs, including ACSL4/LPCAT3/acid-15-lipoxygenase (ALOX15)-dependent enzymatic reactions and Fe2+-dependent non-enzymatic Fenton reactions. In an enzyme-dependent manner, free intracellular PUFAs are first converted into their fatty acyl-CoA form by the enzyme ACSL4 and then esterified into PLs by the enzyme LPCAT3. Then, free and esterified PUFAs are directly catalyzed into their hydroperoxy form by ALOX15 and, thus, promote ferroptosis.[66–70] In addition, cytochrome P450 (CYP450) oxidoreductase can generate lipid peroxidation and trigger ferroptosis, although its specific mechanism is still unclear.[71] These findings suggest that the pharmacological inhibition of the enzymes in LPO-generating pathways, such as ACSL4 and ALOX15, might become a potential strategy for alleviating ferroptosis. In a non-enzymatic manner, intracellular Fe2+ could promote lipid peroxidation and initiate ferroptosis by triggering the Fenton reaction or increasing the catalytic activity of the enzyme ALOX15, which has an iron-containing area and produces ROS.[72] The Fenton reaction is a process in which Fe2+ can react with hydrogen peroxide to produce OH·, OH−, and Fe3+. Then, OH·, assisted by oxygen, could attack the carbon–carbon double bonds of PUFAs and produce lipid hydroperoxides.[73] The process of intracellular iron uptake can be classified into the transferrin (TF)-bound iron uptake pathway, non-TF-bound iron uptake pathway, and heme iron uptake pathway. On the one hand, after the transformation from circulating Fe2+ to Fe3+ by ceruloplasmin, Fe3+-binding TF could further bind the transferrin receptor and be imported to endosomes from invaginated cell membranes. Subsequently, Fe3+ is reduced to Fe2+ by ferrireductase six-transmembrane epithelial antigen of prostate 3 in endosomes, and Fe2+ is released from endosomes to the cytoplasm through solute carrier family 11 member 2 (SLC11A2; also named divalent metal transporter 1) and zinc-and iron-related protein (ZIP) 8/14. On the other hand, non-TF-bound Fe3+ could be reduced to Fe2+ by reductases on the cell surface, such as cytochrome B reductase 1 and prion protein, and then imported to the cytoplasm directly through ZIP8/14.[74,75] In addition, for heme iron, free heme, hemoglobin-binding haptoglobin and heme-binding hemopexin could be imported to the cytoplasm through feline leukemia virus subgroup C receptor-related protein 2/solute carrier family 48 member 1/solute carrier family 46 member 1, cluster of differentiation 163 (CD163), and cluster of differentiation 91 (CD91), respectively, followed by the release of Fe2+ by heme oxygenase 1.[76] Therefore, reducing lipid peroxidation by decreasing iron accumulation might deserve further attention in future research.
Ameliorating lipid peroxidation through antioxidant systems could inhibit ferroptosis and revive cells, including the GSH system, selenium (Se) system, and coenzyme Q (CoQ) system. GPX4, the hub enzyme in these systems, can clear peroxides by catalyzing hydroperoxy PUFAs to their hydroxy forms with the assistance of GSH.[62,66–77] The overexpression or knockdown of GPX4 could modulate the lethality of ferroptosis inducers.[78] How these antioxidant systems fight against ferroptosis is elucidated as follows. First, GSH, consisting of glutamic acid, cysteine, and glycine, mainly exerts its antioxidant function through its sulfhydryl group of cysteine by converting L-OOH to L-OH. Therefore, it is essential to maintain the intracellular cysteine concentration for GSH biosynthesis. The cystine/glutamate antiporter system (system xc-), which is composed of solute carrier family 7 member 11/xCT and solute carrier family 3 member 2, can import extracellular cystine. Then, cystine is reduced to cysteine, and GSH is synthesized under the catalytic function of glutamate-cysteine ligase and glutathione synthetase. Second, Se is a component of selenocysteine at the catalytic site of GPX4.[79] Exogenous supplementation with Se could transcriptionally increase GPX4 expression to protect neurons from ferroptosis.[80] Third, ubiquinol (also named reduced coenzyme Q10), the reduced form of CoQ10, is also regarded as an effective ferroptosis suppressor that traps the lipid peroxyl radicals that mediate lipid peroxidation.[81] Generally, ubiquinol is obtained from CoQ10 through a reduction reaction catalyzed by the nicotinamide adenine dinucleotide-dependent CoQ oxidoreductase ferroptosis suppressor protein 1.[82] The biosynthesis of CoQ10 is regulated by the MVA pathway in which acetyl-CoA is converted into 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA), followed by the synthesis of MVA from HMG-CoA, isopentenyl pyrophosphate (IPP) from MVA, and the final isoprenoid side chain of CoQ10 from IPP.[83] These antioxidant systems synergize to maintain cellular environmental homeostasis by fighting against ROS accumulation, manifesting a druggable potential for alleviating injuries induced by oxidative stress and ferroptosis.
Role of ACSL4 in ferroptosis
ACSL4 is indispensable for ferroptosis execution by initiating the extensive production of LPOs. By generating a genome-wide clustered regularly interspaced short palindromic repeats (CRISPR)-based genetic screen and microarray analysis, researchers found that ACSL4 was significantly downregulated in ferroptosis-resistant cell lines.[84] In addition, ACSL4 knockout (KO) cells were resistant to RSL3, an inhibitor of GPX4 and an inducer of ferroptosis, while ACSL4 overexpression in ferroptosis-resistant cells (such as Lymph Node Carcinoma of the prostate [LNCap] and K562 [human lymphoblast cells of chronic myelogenous leukaemia]) significantly increased their sensitivity to ferroptosis.[84,85] To initiate lipid peroxidation on cell membranes, ACSL4 first catalyzes the conversion of AA into AA-CoA, followed by the esterification of AA-CoA into PE with the assistance of LPCAT3. Then, ALOX15 catalyzes the oxidation of AA-containing PE to its hydroperoxy form (PE-AA-OOH), which acts as an initiating factor of ferroptosis when there is not sufficient GPX4/GSH to conduct reduction reactions.[86] In addition, lipoxygenases (LOXs) catalyze the oxidation of excessive AA-CoA to HETE, such as 5-HETE, which also contributes to ferroptosis.[85] The genetic or pharmacological inhibition of ACSL4 preferentially decreases the synthesis of AA/AdA-CoA and PE-AA/AdA and their doubly and triply oxidized forms, ameliorating the rupture of the outer mitochondrial membrane (OMM).[84,86,87]
Recently, a lipid peroxidation-protein kinase C-beta II (PKCβII)-ACSL4 positive-feedback loop in the execution of ferroptosis was demonstrated. After activation by a slight accumulation of LPOs, phosphorylated and membrane-localized PKCβII activates ACSL4 by phosphorylating its site Thr328, triggering PUFA-containing lipid biosynthesis and promoting the production of LPOs to lethal levels.[88] Tocopherols and tocotrienols of the vitamin E family suppress the activity of LOXs, ameliorate lipid peroxidation and prevent ferroptosis.[86] Cystathionine gamma-lyase-derived hydrogen sulfide can decrease ACSL4 expression and attenuate arachidonate-12-lipoxygenase acetylation to protect against ferroptosis.[89] In addition, ACSL4 KO protected cells from Golgi stress-triggered ferroptosis.[90] In addition, ACSL4 is a collaborative enzyme of GPX4. A significant decrease in ACSL4 expression was observed in GPX4-depleted cells, suggesting a compensatory mechanism to lower ferroptosis sensitivity.[79]
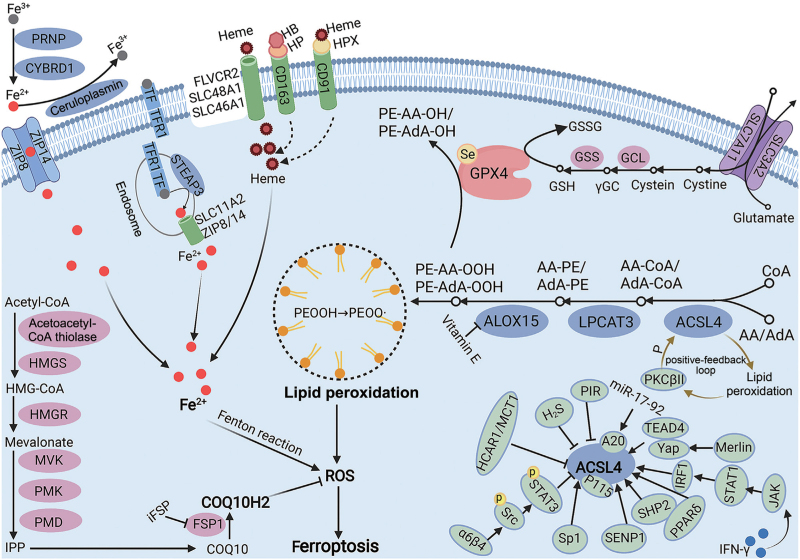
Altogether, ACSL4-initiated lipid peroxidation is fundamental and indispensable for ferroptosis execution [Figure 1], and the role of ACSL4 as a therapeutic target for ferroptosis still needs to be addressed in the future.
Figure 1.
ACSL4 is indispensable for triggering ferroptosis by generating lipid peroxidation. ACSL4 could first catalyze the insertion of CoA into AA and AdA, followed by the esterification of AA-CoA and AdA-CoA into PEs with the assistance of LPCAT3. Then, ALOX15 oxidizes AA-PE and AdA-PE into PE-AA-OOH and PE-AdA-OOH, triggering ferroptosis at cellular membranes. The GPX4/GSH system could reduce PE-AA/AdA-OOH to PE-AA/AdA-OH and, thus, ameliorate lipid peroxidation-mediated ferroptosis. In addition, the Fenton reaction by Fe2+ could generate ROS, while COQ10H2 produced by the MVA pathway could alleviate ROS. Regulators of ACSL4, such as TEAD4, YAP, IRF1, PPARδ, SHP2, SENP, p115, Sp1, α6β4, HCAR1/MCT1, PIR, and A20, are also summarized in the figure. Created with BioRender.com. AA: Arachidonic acid; AA-CoA: Arachidonoyl-coenzyme A (CoA); ACSL4: Acyl-CoA synthase 4; AdA: Adrenic acid; ALOX15: ACSL4/LPCAT3/acid-15-lipoxygenase; CD163: Cluster of differentiation 163; CD91: Cluster of differentiation 91; COQ10: Coenzyme Q10; COQ10H2: Reduced COQ10; CYBRD1: Cytochrome B reductase 1; FLVCR2: Feline leukemia virus subgroup C receptor-related protein 2; FSP1: Ferroptosis suppressor protein 1; GCL: Glutamate cysteine ligase; GPX4: Glutathione peroxidase 4; GSH: Glutathione; GSS: Glutathione synthase; GSSG: Oxidized glutathione; HB: Hemoglobin; HCAR1: Hydroxycarboxylic acid receptor 1; HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A; HMGR: 3-hydroxy-3-methylglutaryl coenzyme-A reductase; HMGS: 3-hydroxy-3-methylglutaryl coenzyme-A synthase; HP: Haptoglobin; HPX: Hemopexin; H2S: Hydrogen sulfide; IPP: Isopentenyl diphosphate; IRF1: Interferon regulatory factor 1; IFN-γ: Interferon γ; JAK: Janus kinase; LPCAT3: Lysophosphatidylcholine acyltransferase 3; MCT1: Monocarboxylate transporter 1; miR: MicroRNA; MVA: Mevalonate; MVK: Mevalonate kinase; P: Phosphorylation; PE: Phosphatidylethanolamine; PIR: Pirin; PMD: Phosphomevalonate decarboxylase; PMK: Phoshpomevalonate kinase; PKCβII: Protein kinase C-beta II; PRNP: Prion protein; PPARδ: Peroxisome proliferator-activated receptor delta; ROS: Reactive oxygen species; SENP: Sentrin-specific protease; SHP2: Src homology-2 domain-containing protein tyrosine phosphatase-2; SLC3A2: Solute carrier family 3 member 2; SLC7A11: Solute carrier family 7 member 11; SLC11A2: Solute carrier family 11 member 2; SLC46A1: Solute carrier family 46 member 1; SLC48A1: Solute carrier family 48 member 1; Sp1: Specificity protein 1; STAT: Signal transducer and activator of transcription; STEAP3: Six-transmembrane epithelial antigen of prostate 3; TEAD4: Transcriptional enhanced associate domain 4; TF: Transferrin; TFR1: Transferrin receptor 1; Yap: Yes-associated protein; ZIP: Zinc-and iron-related protein; γ-GC: γ-glutamylcysteine.
ACSL4 and FA metabolism
Remodeling the PL composition
As a main component of biomembranes, PLs of different species are crucial for maintaining cell function, such as PC, PE, phosphatidylinositol (PI), and phosphatidylserines (PS). A PL usually consists of glycerol/sphingosine, FAs, and phosphate.[69] Since the synthesis of AA-CoA by ACSL4 is the first step in the incorporation of AA into PLs, the inhibition or overexpression of ACSL4 could modulate the proportion of PL-AA in cell membranes. In adipocytes from adipocyte-specific knockout (Ad-KO) mice fed a high-fat diet (HFD), reduced incorporation of AA into all species of PL was observed, while the FA composition of TGs, diacylglycerols, and cardiolipins did not change.[13] In addition, several studies demonstrated a similar reduction in AA-PLs in hepatocytes and human aortic smooth muscle cells (hSMCs) with ACSL4 depletion and HFD consumption. Additionally, LPC and LPE species accumulate.[55,91]
Regulation of eicosanoid biosynthesis
Intracellular free AAs are oxidized to prostaglandins and thromboxanes by cyclooxygenases (COXs); to leukotrienes, lipoxins, and HETEs by LOXs; or to EETs and HETEs by CYP450.[92] ACSL4 contributes to maintaining the intracellular AA levels and, thereby, facilitates the production of eicosanoids. A specific ACSL4 KO in adipocytes from mice fed an HFD resulted in a reduction in AA in intracellular free FA pools.[13] The knockdown of endogenous ACSL4 in interleukin (IL)-1β-treated rat fibroblastic cells or lipopolysaccharide-treated bone marrow-derived macrophages significantly decreased PC and PI species bearing AA and increased the production of prostaglandin E2 (PGE2), prostaglandin D2, and prostaglandin F2α.[12,93] Additionally, a similar enhancement of eicosanoids was observed after treating rat neutrophils, human fibroblastic cells, and hSMCs with triacsin C, an inhibitor of ACSL4.[93,94] Intriguingly, the sustained downregulation of ACSL4 in hSMCs led to a significant reduction in PGE2 release, while short-term ACSL4 inhibition increased the PGE2 levels.[8] In summary, ACSL4 dysfunction may facilitate inflammatory responses by triggering the eicosanoid storm. Furthermore, ACSL4 could regulate the expression of the enzyme COX-2,[95] providing a potential explanation for the enhancement in eicosanoids by ACSL4 inhibition.
Regulation of steroidogenesis
ACSL4 could regulate steroidogenesis by affecting steroidogenic acute regulatory protein (StAR). Steroidogenesis is a biological process in which cholesterol is converted into various steroid hormones.[96] The first and rate-limiting step in steroidogenesis is the conversion of cholesterol into pregnenolone (the precursor of all other steroids).[97] StAR plays a key role in this step by mediating the transfer of cholesterol from the OMM to the inner mitochondrial membrane, where cholesterol is converted into pregnenolone by the enzyme P450scc.[98,99] The expression of StAR is regulated by multiple mechanisms. Among them, cAMP can transduce signals of adrenocorticotropic hormone and luteinizing hormone to activate protein kinase A and then induce the expression of the StAR gene in the nucleus.[100] Notably, under cAMP stimulation, the addition of AA and its oxygenase-derived metabolites, such as EET, 5-HETE, and 5-hydroperoxyeicosatetraenoic acid, could help transcriptionally enhance the expression and activity of StAR and, thereby, increase steroidogenesis.[101–103]
AA is mainly converted into its metabolites by LOX and epoxygenase in the mitochondrial compartment, and the translocation of cytoplasmic AA to mitochondria demands a complex process [Figure 2]. In steroid-producing cells, intracellular free AA is first converted into AA-CoA by ACSL4. Then, AA-CoA binds diazepam-binding inhibitor, which, in turn, translocates to mitochondria through translocator protein at the OMM. Finally, AA-CoA is converted back into free AA and CoA by mitochondrial acyl-CoA thioesterase.[104–106] Under c-AMP stimulation, ACSL4 inhibition could reduce both the mRNA and protein levels of StAR, leading to disturbed steroid biosynthesis.[104,107,108] A possible explanation is that ACSL4 depletion could attenuate the promoting effect of AA and its metabolites on StAR expression by blocking the translocation of AA from the cytoplasm to mitochondria.[104] In addition, ACSL4 deficiency could reduce intracellular cholesteryl ester storage and, thus, decrease steroidogenesis.[14]
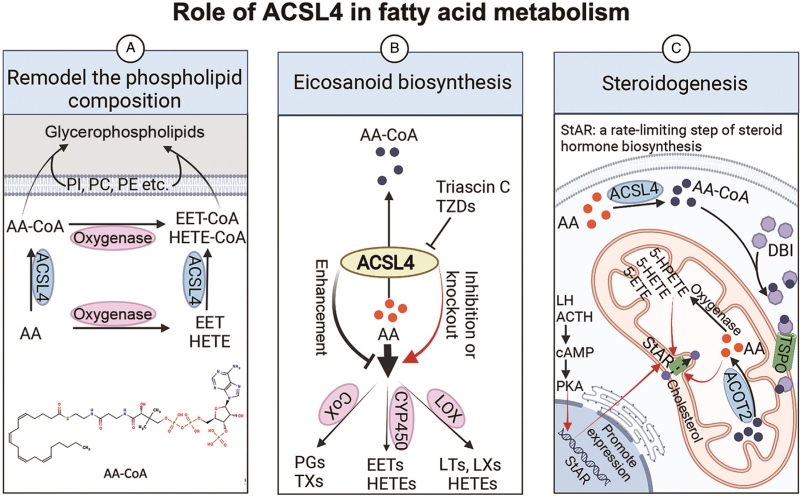
Figure 2.
The role of ACSL4 in FA metabolism. (A) ACSL4 remodels the PL composition of the cellular membrane. (B) The suppression of ACSL4 reduced the esterification of AA into PLs and, thereby, increased the production of eicosanoids. (C) Intracellular free AAs translocate to the intramitochondrial compartment through the ACSL4/DBI/TSPO/ACOT2 pathway and then regulate steroidogenesis by enhancing StAR. Created with BioRender.com. AA: Arachidonic acid; AA-CoA: Arachidonoyl-coenzyme A (CoA); ACOT2: Acyl-CoA thioesterase; ACSL4: Acyl-CoA synthase 4; ACTH: Adrenocorticotropic hormone; cAMP: Cyclic adenosine monophosphate; CoX: Cyclooxygenase; CYP450: Cytochrome P450; DBI: Diazepam-binding inhibitor; EETs: Epoxyeicosatrienoic acids; ETE: Eicosatetraenoic acid; FA: Fatty acid; 5-HETEs: 5-Hydroxyeicosatetraenoic acids; 5-HPETE: 5-Hydroperoxyeicosatetraenoic acid; LOX: Lipoxygenase; LTs: Leukotrienes; LXs: Lipoxins; LH: Luteinizing hormone; PC: Phosphatidylcholine; PE: Phosphatidylethanolamine; PGs: Prostaglandins; PI: Phosphatidylinositol; PKA: Protein kinase A; PLs: Phospholipids; StAR: Steroidogenic acute regulatory protein; TSPO: Translocator protein; TXs: Thromboxanes; TZDs: Thiazolidinediones.
Others
ACSL4 is required for the antitumor T-cell response and proinflammatory cytokine production. ACSL4 silencing in microglial cells inhibits the production of the proinflammatory cytokines tumor necrosis factor α, IL-6, and IL-1β.[109] In addition, ACSL4 KO in CD8+ T cells reduced the T-cell numbers and their specific killing abilities.[110] Moreover, a recent study by Liao et al[111] demonstrated that interferon (IFN)-γ derived from CD8+ T cells could transcriptionally increase ACSL4 expression to upregulate the incorporation of AA into C16 and C18 acyl chain-containing PLs, and AA together with IFN-γ could induce ferroptosis in an ACSL4-dependent manner. Further deletion of ACSL4 promoted tumor growth in immunocompetent mice, but not in immunodeficient mice, indicating that the inhibition of tumor growth by ACSL4 deficiency likely depends on interactions with immune systems.[111]
ACSL4 is also related to insulin secretion. ACSL4 and ACSL3 depletion in human pancreatic islets could change the patterns of FA in PE and PS and inhibit glucose-stimulated insulin release.[9,112] In addition, the inhibition of ACSL4 by miR-130a-3p overexpression could promote the differentiation of neural stem cells into neurons by regulating the protein kinase B/phosphatidylinositol 3-kinase pathway.[38] Additionally, ACSL4 contributes to the formation of viral replication organelles and helps facilitate virus replication.[113] Kung et al[113] reported that inhibiting ACSL4 by rosiglitazone (ROSI) and pioglitazone (PIO) could decrease a load of human enteroviruses and coronaviruses, indicating that ACSL4 is a potential target to counteract viral infection.
ACSL4 in Human Diseases
Recently, increasing evidence has shown the unique role of ASCL4 in human diseases, such as cancers, ischemia/reperfusion (I/R) injuries, and metabolic diseases [Figure 3].
Figure 3.
ACSL4 has been demonstrated to be associated with various human diseases. ACSL4: Acyl-CoA synthase 4; COPD: Chromic obstructive pulmonary disease; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
Cancer
ACSL4 is an executor of ferroptosis in cancer cells due to the upregulated expression of ACSL4 in ferroptosis-sensitive cancer cells (Hepatoma cells [HepG2] and human promyelocytic leukemia cells [HL60]) compared with ferroptosis-resistant cancer cells (LNCaP and K562).[85] Further knockdown of ACSL4 inhibited erastin-induced ferroptosis, while the overexpression of ACSL4 restored the sensitivity of cells to ferroptosis.[85] Moreover, a recent study implicated a potential collaborative role of ACSL4 in tumor immunotherapy. ACSL4 deficiency attenuates the antitumor T-cell response and accelerates tumor progression, and high ACSL4 expression improves the survival of cancer patients treated with immune checkpoint blockade.[111] The mechanism of ACSL4-mediated ferroptosis in cancer cells has been partially clarified in several recent studies. First, the proto-oncogenic coactivator YAP, activated by the Merlin-Hippo pathway, could synergize with TEAD4 to upregulate ACSL4 transcriptionally and, thus, promote ferroptosis in cancer cells.[31] Second, the knockdown of pirin, an iron-binding protein and redox sensor of nuclei, could initiate high-mobility group box-1 (HMGB1)-dependent autophagy and then promote ferroptosis by activating ACSL4.[114] Third, blocking lactate uptake by cancer cells by inhibiting the hydroxycarboxylic acid receptor 1 /monocarboxylate transporter 1 axis could promote ACSL4 expression and lead to ferroptosis.[115] Fourth, pharmacologically inhibiting the production of 5-HETE by zileuton could limit ACSL4-triggered ferroptosis.[85] Altogether, insight into ACSL4-initiated ferroptosis might guide a novel perspective regarding the mechanism of carcinogenesis.
Interestingly, the expression of ACSL4 varies in different cancer types. According to a systematic study of the Oncomine and UALCAN databases, compared with normal tissues, ACSL4 is downregulated in breast cancer (BC), bladder cancer, brain cancer, leukemia, and lung cancer (LC), but upregulated in liver cancer, colorectal cancer, head and neck cancer, myeloma, ovarian cancer, and kidney cancer.[116,117] In addition, a comprehensive analysis of PrognoScan, the Kaplan–Meier mapping database, and Gene Expression Profiling Interactive Analysis revealed that low ACSL4 expression generally predicts a good prognosis across cancers despite the poor prognostic results in several certain cancers.[117] In addition, ACSL4 is negatively correlated with deoxyribonucleic acid (DNA) methylation levels and positively correlated with immune infiltration in most cancers.[117] Although the reason for the differential ACSL4 expression in different cancer types is still unclear, the investigation into the function of ACSL4 in cancer has made rapid progress, and up-to-date findings are summarized as follows.
Hepatocellular carcinoma (HCC)
ACSL4-mediated metabolic reprogramming plays an important role in liver carcinogenesis. The expression of ACSL4 is significantly upregulated in HCC tissues, especially in the high alpha-fetoprotein subtypes.[118–120] Blocking ACSL4 effectively inhibited HCC growth.[121] By CRISPR/Cas9 screening, hexokinase 2 (HK2) is strongly associated with cancer stem cell properties and epigenetically activates the transcription of ACSL4, leading to an increase in FA β-oxidation activity.[121] Chen et al[122]'s study revealed that ACSL4 could prevent the degradation of c-Myc, which could transcriptionally upregulate sterol regulatory element-binding protein 1 (SREBP1) and, thereby, promote its downstream lipogenic enzymes, leading to the aberrant accumulation of intracellular lipid components and HCC progression. In addition, ACSL4 depletion could decrease the phosphorylation of extracellular signal-regulated kinase (ERK), which then directly destabilizes c-Myc by downregulating its phosphorylation at serine 62 or indirectly promotes the ubiquitin degradation of c-Myc by increasing F-box and WD repeat domain-containing 7 (FBW7; an E3 ligase) expression.[118] These results suggest that ACSL4 promotes the progression of HCC via the c-Myc/ERK/FBW7/SREBP1 axis. Wang et al[123] noted that ACSL4 promoted HCC growth by enhancing glucose transporter 1-mediated O-GlcNAcylation, and O-GlcNAcylation could, in turn, increase ACSL4 expression, implying positive feedback between ACSL4 and O-GlcNAcylation. Additionally, ACSL4 could promote the proliferation of HCC cells by activating the mammalian target of rapamycin signaling pathway, which could control multiple metabolic programs, including glycolysis, glutaminolysis, and oxidation of FAs.[123,124] Moreover, the upregulated ACSL4 mRNA expression in apolipoprotein O-silenced HepG2 cells indicates that apolipoprotein O is a potential upstream regulator of ACSL4.[125] miR-211-5p and miR-205 have been identified as two suppressors of ACSL4, and both are downregulated in HCC with a malignant phenotype, resulting in abnormal accumulated cellular cholesterol.[40,41] Conclusively, ACSL4 could reprogram lipid metabolism in HCC by a complex regulating system, probably to meet the endless energy demand of cancer cells.
ACSL4 might serve as a biomarker in the clinical diagnosis and prognosis prediction of HCC. ACSL4 could distinguish HCC tissues from normal tissues with a sensitivity of 93.8% and a specificity of 93.6%.[119] Upregulated ACSL4 is closely associated with a poor prognosis in HCC patients.[118,126] Furthermore, ACSL4 is a good predictor of the sensitivity of the sorafenib response in HCC patients. According to Feng et al[127]'s study, HCC patients with complete or partial response to sorafenib had higher ACSL4 expression, and the ACSL4 levels were negatively related to the half maximal inhibitory concentration values of sorafenib. Further knockdown of ACSL4 significantly abolished sorafenib-induced ferroptotic cancer cell death and tumor growth inhibition.[127] Consistently, synergistic treatment with sorafenib and aspirin had the best antitumor effect in advanced HCC patients with high ACSL4 but low growth arrest and DNA damage-inducible 45 β (GADD45B) expression.[128] In addition, miR-23a-3p, a suppressor of ACLS4, was overexpressed in sorafenib-non-responding HCC. The inhibition of miR-23a-3p rescued ACSL4 expression and promoted the sensitivity of HCC cells to sorafenib.[42] In summary, these results imply that high ACSL4 expression could promote the sorafenib response in HCC; the enhancement of ACSL4 has a druggable potential to ameliorate sorafenib resistance and improve HCC prognosis.
BC
In BC, it has been demonstrated that high ACSL4 expression is correlated with the negative status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) and is associated with aggressive phenotypes, such as increased proliferation and invasion.[84,95,129–131] For example, ACSL4 preferentially showed high expression in ER-negative BC cell lines, such as human breast cancer cells (MDA-MB-231), but low expression in ER-positive BC cell lines, such as breast epithelial cells (MCF-7).[81,129,130] In addition, a meta-analysis of the public gene expression database revealed that high ACSL4 expression was associated with triple- or quadruple-negative BC, characterized by negative androgen receptor (AR), negative ER, negative PR, and negative HER2.[129,132] Intriguingly, exogenous overexpression of ACSL4 in MCF-7 cells could reduce ER expression and estrogen-dependent cell growth[132] and could sensitize these cells to lipid peroxidation-mediated ferroptosis.[84]
Although the detailed mechanism of differential ACSL4 expression in different BC cell lines has not been fully demonstrated, there are some novel clues. Dattilo et al[133] found that the proximal 43 base pairs at the promoter were key regions responsible for increased ACSL4 expression in MDA-MB-231 cells; restoring ERα expression in triple-negative BC cells decreased ACSL4 promoter activity. In addition, the mRNA level of ACSL4 is downregulated by silencing peptidyl arginine deiminase isoform 2 in MCF-7 cells,[134] implicating it as a possible transcriptional activator inducing ACSL4 expression. In addition, the treatment of steroid-starved ER+ BC cells with 17β-estradiol increased the ACSL4 levels, while ER silencing reversed this impact.[135]
Moreover, ACSL4 is closely related to chemotherapeutic and radiotherapeutic resistance in BC. Orlando et al[136] found that chemotherapeutic agents, such as cisplatin and doxorubicin, had an unsatisfactory inhibitory effect on BC cell growth with high ACSL4 expression, while this effect was counteracted when ACSL4 was inhibited. The mechanism further revealed that ACSL4 was able to upregulate cellular transporters, such as ATP-binding cassette sub-family G member 2, ATP-binding cassette transporter family class C4, and ATP-binding cassette transporter family class C8, which cause the efflux of chemotherapeutic drugs.[136] In contrast, another study showed that in BC patients receiving neoadjuvant chemotherapy, high ACSL4 expression could serve as an independent predictive factor of a better pathological complete response and better overall survival,[95] revealing the controversial role of ACSL4 in the response to chemotherapeutic drugs. Additionally, ACSL4 expression was significantly elevated in radioresistant BC cells, and inhibiting the ACSL4-forkhead box protein M1 pathway could improve the response of BC cells to irradiation.[137,138] These results show that targeting ACSL4 may help ameliorate chemotherapeutic and radiotherapeutic resistance. However, whether the collaborative administration of ACSL4 inhibitors with chemotherapy or radiotherapy could benefit BC patients demands further evaluation.
Prostate cancer (PC)
ACSL4 may be not only an indicator of PC but also a therapeutic target in castration-resistant PC (CRPC) treatment. In contrast to adjacent benign epithelial cells, ACSL4 is highly expressed in malignant PC cells, and ACSL4 is particularly higher in CRPC than in hormone-naive PC.[139] Moreover, according to the study of Ma et al[140], since the AR suppresses ACSL4 expression by binding its promoter, ACSL4 is highly expressed in AR pathway-independent PC and promotes its proliferation, migration, and invasion. These results suggest that ACSL4 is a potential therapeutic target for one-third of PC patients who develop androgen deprivation therapy resistance.
Recently, Castillo et al[57] discovered a new inhibitor of ACSL4–PRGL493. This inhibitor can inhibit de novo steroid synthesis, suppress PC tumor growth in animal models, and sensitize PC cells to chemotherapeutic and hormonal treatment.[57] In addition, the long non-coding RNA nuclear-enriched abundant transcript 1 (lncRNA NEAT1) could promote docetaxel resistance in PC cells by elevating ACSL4 expression via the inhibition of miR-34a-5p and miR-204-5p.[141]
LC
Several recent studies have demonstrated that ACSL4 is a suppressor of LC that could inhibit the survival, invasion, and migration of cancer cells and accelerate their ferroptosis.[142] According to the data from The Cancer Genome Atlas, Oncomine, PrognoScan, and validation samples, compared with normal tissues, ACSL4 was downregulated in lung adenocarcinoma, and low ACSL4 expression predicts poor survival in LC.[83,142] The lncRNA NEAT1 could promote ferroptosis sensitivity in non-small cell lung cancer (NSCLC) by upregulating ACSL4 expression.[143] The endoplasmic reticulum stress induced by the LC microenvironment could decrease ACSL4 expression and FA uptake, but did not change the intracellular ROS levels.[144] In addition, curcumin, accompanied by elevated ACSL4 levels, could induce ferroptosis by activating autophagy in NSCLC for therapeutic effects.[145] Remarkably, the suppression of ACSL4 by bone morphogenetic protein 4 depletion attenuated the resistance effect and restored FAs, triglycerides, and CEs in gefitinib-resistant NSCLC cells,[146] indicating that ACSL4-mediated FA metabolism might play a significant role in the maintenance of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) resistance.
Other cancer types
Strikingly, ACSL4, in synergism with its downstream lipid metabolic reprogramming and ferroptosis execution, is also involved in colon cancer, pancreatic cancer, glioma, cervical cancer, and renal cancer. In colon cancer, several anticancer molecules and extracts can exert cancer cell-killing effects by upregulating ACSL4 to promote ferroptosis. High expression of cytoglobin, which maintains oxygen homeostasis, could promote ferroptotic colon cancer cell death by increasing the expression of the P53-YAP1-ACSL4 axis.[147] Apatinib, a vascular endothelial growth factor receptor (VEGFR) inhibitor in cancer treatment, can upregulate ACSL4 and initiate ferroptosis in colon cancer cells.[148] Bromelain, a mixture of enzymes from pineapple, could remarkably upregulate ACSL4 and exert cytotoxic effects in K-Ras mutant colon cancer cells.[149] In addition, the antiproliferative effect of ACSL4-mediated ferroptosis is observed in glioma and glioblastoma. ACSL4 is downregulated in glioma, and the exogenous overexpression of ACSL4 in glioma cells could mediate a reduction in its viability.[150] Additionally, in contrast to isocitrate dehydrogenase (IDH) 1 wild-type glioma, ACSL4 represents lower protein expression in the IDH1 mutant type, which is more responsive to interventions.[151] Intriguingly, ACSL4-mediated ferroptosis could promote necrosis in glioblastoma progression, and ACSL4 depletion by small hairpin RNA significantly diminished necrosis and aggressiveness in tumors.[152] Dihydrotanshinone I and dihydroartemisinin can inhibit human glioma and glioblastoma progression by activating ACSL4-mediated ferroptosis,[153,154] which might provide novel treatment prospects. Furthermore, a bioinformatics analysis identified ACSL4 as a significantly upregulated gene in pancreatic cancer.[155] Ye et al[156] found that the abrogation of adenosine 5′-diphosphate ribosylation factor 6 (ARF6) could increase the ACSL4 protein levels and, thus, promote RSL3-induced ferroptosis in pancreatic cancer cells, and ROSI inhibiting ACSL4 could abolish this effect, indicating that ARF6 is an upstream regulator of ACSL4. Similarly, in pancreatic ductal adenocarcinoma, the suppression of ACSL4 expression by upregulated tyrosine phosphatase mitochondria 1 (protein tyrosine phosphatase, mitochondrial 1) could inhibit ferroptosis.[157] Moreover, ACSL4 is also involved in cervical cancer, although its role has not been clearly elucidated. The circular RNA LIM domain only 1 (LOM1) was found to be downregulated in cervical cancer tissues and could function as a competing endogenous RNA by sponging miR-4192 to repress the target gene ACSL4.[158] Oleanolic acid treatment in cervical carcinoma cells and xenograft models could promote ACSL4 expression and, thus, impair cancer development, and the inhibition of ACSL4 by small interfering RNA (siRNA) could counteract its antitumor effect, which might depend on ACSL4-mediated ferroptosis.[159] In addition, in renal cell carcinoma (RCC) patients, ACSL4 expression was notably elevated and indicated a poor prognosis.[44] Zinc oxide nanoparticles can inhibit the tumorigenesis of RCC by modulating lipid metabolism via miR-454-3p/ACSL4 axis.[44] Altogether, we identified the profound and indispensable function of ACSL4 in various cancer types; insight into targeting ACSL4 provides a novel strategy in cancer treatment.
ACSL4 and I/R-induced injuries
IR-induced injuries are caused by sudden temporary ischemia and reperfusion of the blood flow to organs, resulting in organ damage by a robust inflammatory and oxidative stress response. High ACSL4 expression and ferroptosis have been widely observed after I/R in different organs, such as the cerebrum, lung, intestine, heart, and kidney.[30,160–163] During cerebral I/R, thrombin was regarded as a potential contributor to ferroptosis-mediated injuries by promoting AdA mobilization and its subsequent esterification by ACSL4.[160] The lncRNA lncAABR07025387.1 could negatively regulate the miR-205 expression and, hence, upregulate ACSL4-mediated ferroptosis in I/R injuries.[161]
Recent studies revealed that several agents inhibiting ACSL4 could protect against I/R injury. Pretreatment with baicalin, a natural flavonoid glycoside, prevented myocardial injury by inhibiting ACSL4-mediated ferroptosis in a myocardial I/R rat model. However, in myocardial cells subjected to oxygen-glucose deprivation/reoxygenation, baicalin could no longer show a protective effect.[162] Inhibiting ACSL4 by ROSI before ischemia could diminish ferroptotic damage in IR-injured lung tissue.[163] In addition, treatment with ROSI after ischemia but before reperfusion could alleviate ferroptosis-induced intestinal injury.[30] These results suggest that ACSL4 inhibitor administration before I/R is a prospective approach for injury prevention, although further clinical research is needed for confirmation.
ACSL4 and metabolic diseases
Upregulated ACSL4 expression and accelerated ferroptosis are often observed in diabetes and its complications.[164,165] The inhibition of ACSL4 by ROSI attenuated ferroptosis, improved kidney function, and promoted the survival rate in diabetic kidney disease (DKD) mice.[164] In addition, HMGB1 suppression could decrease ACSL4 expression, prevent ROS, and restore cellular proliferation in erastin- and high glucose (HG)-treated mesangial cells,[166] a possible hint that HMGB1 is an upstream regulator of ACSL4-mediated ferroptosis in DKD. Moreover, ACSL4-mediated ferroptosis of retinal pigment epithelial cells contributes to diabetic retinopathy (DR).[165] Glia maturation factor-β protein is secreted and accumulates in vitreous body exposed to HG, which could prevent chaperone-mediated autophagic degradation of ACSL4 in the lysosome by impairing the assembly of the lysosome or alkalinizing the lysosome.[165] These results indicate a significant role of ACSL4 in diabetic complications, and the underlying mechanism needs to be further elucidated.
Similarly, emerging evidence has revealed a close relationship between liver diseases and ACSL4. ACSL4 was upregulated in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).[167,168] The hypomethylated CpG site of ACSL4 was associated with an increased risk of NAFLD and NASH.[169] In addition, the suppression of ACSL4 by ROSI or siRNA significantly alleviated arsenic-induced NASH and ferroptosis by diminishing the 5-HETE content.[168] Nevertheless, the substantial mechanism of ACSL4 in NAFLD and NASH remains unclear.
ACSL4 is also involved in cardiovascular and cerebrovascular injuries. By analyzing 40 human coronary artery specimens, ACSL4 was found to be upregulated in the advanced stage of atherosclerosis and positively related to the severity of atherosclerosis.[170] ACSL4-miR5905p-IL1B was proposed to be a pathway that affects the progression of acute myocardial infarction disease.[171] In addition, the depletion of circular RNA Carm1 could protect against acute cerebral infarction by binding miRNA-3098-3p to regulate ACSL4,[42] indicating that Carm1 has a druggable potential to attenuate cerebrovascular injuries.
A Potential Therapeutic Target
The antitumor resistance to chemotherapy and radiotherapy related to ACSL4 expression differs in different cancer types, indicating a complicated role of ACSL4 in cancer treatment. For example, clinical evidence demonstrates that high ACSL4 expression predicts a better antitumor effect of sorafenib in HCC.[127,128] However, PC and LC with high ACSL4 expression represent high resistance to chemotherapeutic and EGFR-TKI treatment, respectively.[57,146] On the one hand, abnormal lipid biosynthesis and enhanced extracellular lipid uptake occur to meet the energy demand of unrestricted cancer cell growth, indicating that ACSL4 inhibition might inhibit cancer cell growth.[172] On the other hand, ACSL4-mediated ferroptosis contributes to exacerbated cancer cell death, indicating that high ACSL4 expression might help cancer treatment.[173] Therefore, the comprehensive function of ACSL4 in cancer progression and treatment remains controversial, and its specific mechanism needs to be further clarified.
In addition, as described above, inhibiting ACSL4 is a practical therapeutic strategy to attenuate ferroptosis-mediated tissue damage in acute kidney injury, atherosclerosis, DKD, DR, NASH, NAFLD, and I/R. The suppression of ACSL4 by TZDs has shown druggable potential in ameliorating kidney dysfunction in DKD. TZDs, agonists of PPARγ, selectively inhibit ACSL4 among the ACSL family.[54] By using distinct TZDs, such as ROSI, PIO, and troglitazone, ferroptosis and lipid peroxidation induced by RSL3 in cells were all prevented.[55] Further research revealed that ROSI could attenuate ferroptosis in renal tubular cells and, thus, improve kidney function in DKD mice.[140] In addition, several new compounds inhibiting ACSL4 have emerged in recent years. Abemaciclib, an oral cyclin-dependent kinase 4/6 inhibitor, is a potent and selective ACSL4 inhibitor that could surprisingly ameliorate the symptoms of NAFLDs.[56] PRGL493, also a newly found compound inhibiting ACSL4 and its downstream steroid synthesis, could inhibit PC cell proliferation.[57]
Although the treatment benefit of these ACSL4 inhibitors has been elucidated in many studies, a series of difficulties in their application in clinical practice exist. First, since ACSL4 is a vital enzyme that activates PUFAs for their subsequent metabolism, whether a lack of ACSL4 could worsen the metabolic disorder remains unexplored. Second, how to suppress ACSL4 selectively without affecting the biological function of other molecules is also a major obstacle. For example, triacsin C could sensitize colorectal carcinoma cells to chemotherapy.[174] Triacsin C is a natural intracellular ACSL inhibitor from Streptomyces aureofaciens that can suppress the activity of the whole ACSL family,[52,53] which might impair the essential function of other ACSL members and produce excessive damage. Third, testing the efficacy and safety of these inhibitors in clinical research is also an unmet need. Collectively, much more research is needed before it will be possible to target ACSL4 in human diseases.
Conclusions
In summary, ACSL4 is a key enzyme that mainly activates AA and AdA by inserting CoA into them, which is a basic step necessary for their metabolism. ACSL4 is also indispensable for FA metabolism regulation and ferroptosis execution by initiating PL peroxidation. Several recent studies also demonstrated a close interaction between ACSL4 and immunity, implicating ACSL4 as a potential target to promote antitumor immunity by triggering ferroptosis. However, the detailed mechanism and whether this is available for clinical practice demand further elucidation. In addition, ACSL4-mediated ferroptosis and lipid metabolism disorders are involved in I/R-induced injuries, AKI, DR, DKD, NASH, NAFLD, and atherosclerosis. Due to the complicated role of ACSL4 in these different diseases, targeting ACSL4 for disease treatment is prospective but challenging.
Acknowledgements
We thank YC Han, H Zhao, JF Yang, JH Liu, TY Duan, Q Zhao, LY Sun, YY Xi, LJ Xu, CR Li, Y Liu, and CY Zhao for their helpful suggestions concerning the writing of this review.
Funding
This work was supported by the Key Program of General Program of the National Natural Science Foundation of China (NSFC) (No. 81730018) and the Natural Science Foundation of Hunan Province (No. 2021JC0003).
Conflicts of interest
None.
Footnotes
How to cite this article: Ding K, Liu C, Li L, Yang M, Jiang N, Luo S, Sun L. Acyl-CoA synthase ACSL4: an essential target in ferroptosis and fatty acid metabolism. Chin Med J 2023;136:2521–2537. doi: 10.1097/CM9.0000000000002533
References
- 1.de Carvalho CCCR, Caramujo MJ. The various roles of fatty acids. Molecules 2018; 23:2583.doi: 10.3390/molecules23102583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Y, Zhou J, Hooi SC, Jiang YM, Lu GD. Fatty acid activation in carcinogenesis and cancer development: essential roles of long-chain acyl-CoA synthetases. Oncol Lett 2018; 16:1390–1396. doi: 10.3892/ol.2018.8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steensels S, Ersoy BA. Fatty acid activation in thermogenic adipose tissue. Biochim Biophys Acta Mol Cell Biol Lipids 2019; 1864:79–90. doi: 10.1016/j.bbalip.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg SJ, Morgenthaler J, Heinzer AK, Smith KD, Watkins PA. Very long-chain acyl-CoA synthetases. Human “bubblegum” represents a new family of proteins capable of activating very long-chain fatty acids. J Biol Chem 2000; 275:35162–35169. doi: 10.1074/jbc.M006403200. [DOI] [PubMed] [Google Scholar]
- 5.Quan J, Bode AM, Luo X. ACSL family: the regulatory mechanisms and therapeutic implications in cancer. Eur J Pharmacol 2021; 909:174397.doi: 10.1016/j.ejphar.2021.174397. [DOI] [PubMed] [Google Scholar]
- 6.Yan S, Yang XF, Liu HL, Fu N, Ouyang Y, Qing K. Long-chain acyl-CoA synthetase in fatty acid metabolism involved in liver and other diseases: an update. World J Gastroenterol 2015; 21:3492–3498. doi: 10.3748/wjg.v21.i12.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radif Y, Ndiaye H, Kalantzi V, Jacobs R, Hall A, Minogue S, et al. The endogenous subcellular localizations of the long chain fatty acid-activating enzymes ACSL3 and ACSL4 in sarcoma and breast cancer cells. Mol Cell Biochem 2018; 448:275–286. doi: 10.1007/s11010-018-3332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golej DL, Askari B, Kramer F, Barnhart S, Vivekanandan-Giri A, Pennathur S, et al. Long-chain acyl-CoA synthetase 4 modulates prostaglandin E2 release from human arterial smooth muscle cells. J Lipid Res 2011; 52:782–793. doi: 10.1194/jlr.M013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klett EL, Chen S, Edin ML, Li LO, Ilkayeva O, Zeldin DC, et al. Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol Chem 2013; 288:21618–21629. doi: 10.1074/jbc.M113.481077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimbara-Matsubayashi S, Kuwata H, Tanaka N, Kato M, Hara S. Analysis on the substrate specificity of recombinant human acyl-CoA synthetase ACSL4 variants. Biol Pharm Bull 2019; 42:850–855. doi: 10.1248/bpb.b19-00085. [DOI] [PubMed] [Google Scholar]
- 11.Gan B. ACSL4, PUFA, and ferroptosis: new arsenal in anti-tumor immunity. Signal Transduct Target Ther 2022; 7:128.doi: 10.1038/s41392-022-01004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwata H, Nakatani E, Shimbara-Matsubayashi S, Ishikawa F, Shibanuma M, Sasaki Y, et al. Long-chain acyl-CoA synthetase 4 participates in the formation of highly unsaturated fatty acid-containing phospholipids in murine macrophages. Biochim Biophys Acta Mol Cell Biol Lipids 2019; 1864:1606–1618. doi: 10.1016/j.bbalip.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Killion EA, Reeves AR, El Azzouny MA, Yan Q-W, Surujon D, Griffin JD, et al. A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction. Mol Metab 2018; 9:43–56. doi: 10.1016/j.molmet.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Hao X, Han L, Yan Z, Shen WJ, Dong D, et al. Tissue-specific ablation of ACSL4 results in disturbed steroidogenesis. Endocrinology 2019; 160:2517–2528. doi: 10.1210/en.2019-00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccini M, Vitelli F, Bruttini M, Pober BR, Jonsson JJ, Villanova M, et al. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics 1998; 47:350–358. doi: 10.1006/geno.1997.5104. [DOI] [PubMed] [Google Scholar]
- 16.Meloni I, Muscettola M, Raynaud M, Longo I, Bruttini M, Moizard M-P, et al. FACL4, encoding fatty acid-CoA ligase 4, is mutated in nonspecific X-linked mental retardation. Nat Genet 2002; 30:436–440. doi: 10.1038/ng857. [DOI] [PubMed] [Google Scholar]
- 17.Ropers H-H, Hamel BCJ. X-linked mental retardation. Nat Rev Genet 2005; 6:46–57. doi: 10.1038/nrg1501. [DOI] [PubMed] [Google Scholar]
- 18.Ruść A, Sieczkowska H, Krzęcio E, Antosik K, Zybert A, Koćwin-Podsiadła M, et al. The association between acyl-CoA synthetase (ACSL4) polymorphism and intramuscular fat content in (Landrace × Yorkshire) × Duroc pigs. Meat Sci 2011; 89:440–443. doi: 10.1016/j.meatsci.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Corominas J, Ramayo-Caldas Y, Castelló A, Muñoz M, Ibáñez-Escriche N, Folch JM, et al. Evaluation of the porcine ACSL4 gene as a candidate gene for meat quality traits in pigs. Anim Genet 2012; 43:714–720. doi: 10.1111/j.1365-2052.2012.02335.x. [DOI] [PubMed] [Google Scholar]
- 20.Mercadé A, Estellé J, Pérez-Enciso M, Varona L, Silió L, Noguera JL, et al. Characterization of the porcine acyl-CoA synthetase long-chain 4 gene and its association with growth and meat quality traits. Anim Genet 2006; 37:219–224. doi: 10.1111/j.1365-2052.2006.01436.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen JN, Jiang YZ, Cen WM, Xing SH, Zhu L, Tang GQ, et al. Distribution of H-FABP and ACSL4 gene polymorphisms and their associations with intramuscular fat content and backfat thickness in different pig populations. Genet Mol Res 2014; 13:6759–6772. doi: 10.4238/2014.August.28.20. [DOI] [PubMed] [Google Scholar]
- 22.Kotronen A, Yki-Järvinen H, Aminoff A, Bergholm R, Pietiläinen KH, Westerbacka J, et al. Genetic variation in the ADIPOR2 gene is associated with liver fat content and its surrogate markers in three independent cohorts. Eur J Endocrinol 2009; 160:593–602. doi: 10.1530/EJE-08-0900. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Murphy KJ, McIntyre TM, Zimmerman GA, Prescott SM. Expression of fatty acid-CoA ligase 4 during development and in brain. FEBS Lett 2000; 467:263–267. doi: 10.1016/s0014-5793(00)01159-5. [DOI] [PubMed] [Google Scholar]
- 24.Küch E-M, Vellaramkalayil R, Zhang I, Lehnen D, Brügger B, Sreemmel W, et al. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim Biophys Acta 2014; 1841:227–239. doi: 10.1016/j.bbalip.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Brown CW, Amante JJ, Goel HL, Mercurio AM. The α6β4 integrin promotes resistance to ferroptosis. J Cell Biol 2017; 216:4287–4297. doi: 10.1083/jcb.201701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen P, Kan CFK, Singh AB, Rius M, Kraemer FB, Sztul E, et al. Identification of p115 as a novel ACSL4 interacting protein and its role in regulating ACSL4 degradation. J Proteomics 2020; 229:103926.doi: 10.1016/j.jprot.2020.103926. [DOI] [PubMed] [Google Scholar]
- 27.Xiao FJ, Zhang D, Wu Y, Jia QH, Zhang L, Li YX, et al. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem Biophys Res Commun 2019; 515:448–454. doi: 10.1016/j.bbrc.2019.05.147. [DOI] [PubMed] [Google Scholar]
- 28.Kan CFK, Singh AB, Stafforini DM, Azhar S, Liu J. Arachidonic acid downregulates acyl-CoA synthetase 4 expression by promoting its ubiquitination and proteasomal degradation. J Lipid Res 2014; 55:1657–1667. doi: 10.1194/jlr.M045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlando U, Cooke M, Cornejo Maciel F, Papadopoulos V, Podestá EJ, Maloberti P. Characterization of the mouse promoter region of the acyl-CoA synthetase 4 gene: role of Sp1 and CREB. Mol Cell Endocrinol 2013; 369:15–26. doi: 10.1016/j.mce.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 2019; 26:2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 2019; 572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kan CFK, Singh AB, Dong B, Shende VR, Liu J. PPARδ activation induces hepatic long-chain acyl-CoA synthetase 4 expression in vivo and in vitro. Biochim Biophys Acta 2015; 1851:577–587. doi: 10.1016/j.bbalip.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai YT, Xiao FJ, Wang H, Ge RL, Wang LS. Hypoxia protects H9c2 cells against ferroptosis through SENP1-mediated protein DeSUMOylation. Int J Med Sci 2021; 18:1618–1627. doi: 10.7150/ijms.50804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooke M, Orlando U, Maloberti P, Podestá EJ, Cornejo Maciel F. Tyrosine phosphatase SHP2 regulates the expression of acyl-CoA synthetase ACSL4. J Lipid Res 2011; 52:1936–1948. doi: 10.1194/jlr.M015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma LL, Liang L, Zhou D, Wang SW. Tumor suppressor miR-424-5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma 2011; 68:165–173. doi: 10.4149/neo_2020_200707N705. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Oh J, Kim YI, Choe SK, Chun CH, Jin EJ, et al. Suppression of ABCD2 dysregulates lipid metabolism via dysregulation of miR-141: ACSL4 in human osteoarthritis. Cell Biochem Funct 2018; 36:366–376. doi: 10.1002/cbf.3356. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Hussain MM. Human microRNA-548p decreases hepatic apolipoprotein B secretion and lipid synthesis. Arterioscler Thromb Vasc Biol 2017; 37:786–793. doi: 10.1161/ATVBAHA.117.309247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W, Li X, Ding N, Teng J, Zhang S, Zhang Q, et al. miR-34a regulates adipogenesis in porcine intramuscular adipocytes by targeting ACSL4. BMC Genet 2020; 21:33.doi: 10.1186/s12863-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Shan BQ, Zhao HY, He H, Tian ML, Cheng X, et al. miR-130a-3p regulates neural stem cell differentiation in vitro by targeting Acsl4. J Cell Mol Med 2022; 26:2717–2727. doi: 10.1111/jcmm.17285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin X, Zhang J, Lin Y, Sun XM, Zhang JN, Cheng ZQ. Identification of miR-211-5p as a tumor suppressor by targeting ACSL4 in hepatocellular carcinoma. J Transl Med 2020; 18:326.doi: 10.1186/s12967-020-02494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui M, Xiao Z, Sun B, Wang Y, Zheng M, Ye L, et al. Involvement of cholesterol in hepatitis B virus X protein-induced abnormal lipid metabolism of hepatoma cells via up-regulating miR-205-targeted ACSL4. Biochem Biophys Res Commun 2014; 445:651–655. doi: 10.1016/j.bbrc.2014.02.068. [DOI] [PubMed] [Google Scholar]
- 42.Lu Y, Chan YT, Tan HY, Zhang C, Guo W, Xu Y, et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res 2022; 41:3.doi: 10.1186/s13046-021-02208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao R, Liu H. Depletion of mmu_circ_0001751 (circular RNA Carm1) protects against acute cerebral infarction injuries by binding with microRNA-3098-3p to regulate acyl-CoA synthetase long-chain family member 4. Bioengineered 2022; 13:4063–4075. doi: 10.1080/21655979.2022.2032971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou X, Cao T. Zinc oxide nanoparticle inhibits tumorigenesis of renal cell carcinoma by modulating lipid metabolism targeting miR-454-3p to repressing metabolism enzyme ACSL4. J Oncol 2022; 2022:2883404.doi: 10.1155/2022/2883404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen B, Wang H, Lv C, Mao C, Cui Y. Long non-coding RNA H19 protects against intracerebral hemorrhage injuries via regulating microRNA-106b-5p/acyl-CoA synthetase long chain family member 4 axis. Bioengineered 2021; 12:4004–4015. doi: 10.1080/21655979.2021.1951070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng Y, Xiang H, Chen C, Zheng R, Chai J, Peng J, et al. miR-224 impairs adipocyte early differentiation and regulates fatty acid metabolism. Int J Biochem Cell Biol 2013; 45:1585–1593. doi: 10.1016/j.biocel.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Bao C, Zhang J, Xian SY, Chen F. MicroRNA-670-3p suppresses ferroptosis of human glioblastoma cells through targeting ACSL4. Free Radic Res 2021; 55:853–864. doi: 10.1080/10715762.2021.1962009. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Zhi F, Lun W, Deng Q, Zhang W. Baicalin inhibits PDGF-BB-induced hepatic stellate cell proliferation, apoptosis, invasion, migration and activation via the miR-3595/ACSL4 axis. Int J Mol Med 2018; 41:1992–2002. doi: 10.3892/ijmm.2018.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gubern C, Camós S, Ballesteros I, Rodríguez R, Romera VG, Cañadas R, et al. miRNA expression is modulated over time after focal ischaemia: Up-regulation of miR-347 promotes neuronal apoptosis. FEBS J 2013; 280:6233–6246. doi: 10.1111/febs.12546. [DOI] [PubMed] [Google Scholar]
- 50.Zhou J, Xiao C, Zheng S, Wang Q, Zhu H, Zhang Y, et al. MicroRNA-214-3p aggravates ferroptosis by targeting GPX4 in cisplatin-induced acute kidney injury. Cell Stress Chaperones 2022; 27:325–336. doi: 10.1007/s12192-022-01271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Z, Yin Y, Jiang J, Yan C, Wang Y, Wang D, et al. Exosomal miR-142-3p secreted by hepatitis B virus (HBV)-hepatocellular carcinoma (HCC) cells promotes ferroptosis of M1-type macrophages through SLC3A2 and the mechanism of HCC progression. J Gastrointest Oncol 2022; 13:754–767. doi: 10.21037/jgo-21-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dechandt CRP, Zuccolotto-Dos-Reis FH, Teodoro BG, Fernandes AMAP, Eberlin MN, Kettelhut IC, et al. Triacsin C reduces lipid droplet formation and induces mitochondrial biogenesis in primary rat hepatocytes. J Bioenerg Biomembr 2017; 49:399–411. doi: 10.1007/s10863-017-9725-9. [DOI] [PubMed] [Google Scholar]
- 53.Kim Y, George D, Prior AM, Prasain K, Hao S, Le DD, et al. Novel triacsin C analogs as potential antivirals against rotavirus infections. Eur J Med Chem 2012; 50:311–318. doi: 10.1016/j.ejmech.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson MA, Mattison DR, Azoulay L, Krewski D. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future. Crit Rev Toxicol 2018; 48:52–108. doi: 10.1080/10408444.2017.1351420. [DOI] [PubMed] [Google Scholar]
- 55.Askari B, Kanter JE, Sherrid AM, Golej DL, Bender AT, Liu J, et al. Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-gamma-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes 2007; 56:1143–1152. doi: 10.2337/db06-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan J, Wang Z, Duan R, Yang C, Zhao R, Feng Q, et al. Therapeutic targeting of hepatic ACSL4 ameliorates NASH in mice. Hepatology 2022; 75:140–153. doi: 10.1002/hep.32148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castillo AF, Orlando UD, Maloberti PM, Prada JG, Dattilo MA, Solano AR, et al. New inhibitor targeting acyl-CoA synthetase 4 reduces breast and prostate tumor growth, therapeutic resistance and steroidogenesis. Cell Mol Life Sci 2021; 78:2893–2910. doi: 10.1007/s00018-020-03679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Modi HR, Basselin M, Rapoport SI. Valnoctamide, a non-teratogenic amide derivative of valproic acid, inhibits arachidonic acid activation in vitro by recombinant acyl-CoA synthetase-4. Bipolar Disord 2014; 16:875–880. doi: 10.1111/bdi.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuohetahuntila M, Spee B, Kruitwagen HS, Wubbolts R, Brouwers JF, van de Lest CH, et al. Role of long-chain acyl-CoA synthetase 4 in formation of polyunsaturated lipid species in hepatic stellate cells. Biochim Biophys Acta 2015; 1851:220–230. doi: 10.1016/j.bbalip.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Gijón MA, Riekhof WR, Zarini S, Murphy RC, Voelker DR. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J Biol Chem 2008; 283:30235–30245. doi: 10.1074/jbc.M806194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012; 149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy 2021; 17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol 2016; 26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ 2016; 23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017; 171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 2016; 113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, et al. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci 2017; 108:2187–2194. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014; 2014:360438.doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang B, Tontonoz P. Phospholipid remodeling in physiology and disease. Annu Rev Physiol 2019; 81:165–188. doi: 10.1146/annurev-physiol-020518-114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol 2015; 10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 2020; 16:302–309. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaitidis P, Adel S, Anton M, Heydeck D, Kuhn H, Horn T. Lipoxygenase pathways in Homo neanderthalensis: functional comparison with Homo sapiens isoforms. J Lipid Res 2013; 54:1397–1409. doi: 10.1194/jlr.M035626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haschka D, Hoffmann A, Weiss G. Iron in immune cell function and host defense. Semin Cell Dev Biol 2021; 115:27–36. doi: 10.1016/j.semcdb.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Lane DJR, Merlot AM, Huang MLH, Bae DH, Jansson PJ, Sahni S, et al. Cellular iron uptake, trafficking and metabolism: key molecules and mechanisms and their roles in disease. Biochim Biophys Acta 2015; 1853:1130–1144. doi: 10.1016/j.bbamcr.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 75.Ji C, Kosman DJ. Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J Neurochem 2015; 133:668–683. doi: 10.1111/jnc.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh N, Ahmad Z, Baid N, Kumar A. Host heme oxygenase-1: friend or foe in tackling pathogens? IUBMB Life 2018; 70:869–880. doi: 10.1002/iub.1868. [DOI] [PubMed] [Google Scholar]
- 77.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res 2021; 31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014; 156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 2018; 172:409–422.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 80.Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 2019; 177:1262–1279. e25. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 81.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019; 575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 82.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019; 575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bathaie SZ, Ashrafi M, Azizian M, Tamanoi F. Mevalonate pathway and human cancers. Curr Mol Pharmacol 2017; 10:77–85. doi: 10.2174/1874467209666160112123205. [DOI] [PubMed] [Google Scholar]
- 84.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 2017; 13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun 2016; 478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 86.Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic Pes navigate cells to ferroptosis. Nat Chem Biol 2017; 13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuwata H, Tomitsuka Y, Yoda E, Hara S. Role of ACSL4 in the chemical-induced cell death in human proximal tubule epithelial HK-2 cells. Biosci Rep 2022; 42:BSR20212433.doi: 10.1042/BSR20212433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang HL, Hu BX, Li ZL, Du T, Shan JL, Ye ZP, et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol 2022; 24:88–98. doi: 10.1038/s41556-021-00818-3. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Yu R, Wu L, Yang G. Hydrogen sulfide guards myoblasts from ferroptosis by inhibiting ALOX12 acetylation. Cell Signal 2021; 78:109870.doi: 10.1016/j.cellsig.2020.109870. [DOI] [PubMed] [Google Scholar]
- 90.Alborzinia H, Ignashkova TI, Dejure FR, Gendarme M, Theobald J, Wölfl S, et al. Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun Biol 2018; 1:210.doi: 10.1038/s42003-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh AB, Kan CFK, Kraemer FB, Sobel RA, Liu J. Liver-specific knockdown of long-chain acyl-CoA synthetase 4 reveals its key role in VLDL-TG metabolism and phospholipid synthesis in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 2019; 316:E880–E894. doi: 10.1152/ajpendo.00503.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol 2004; 24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 93.Kuwata H, Yoshimura M, Sasaki Y, Yoda E, Nakatani Y, Kudo I, et al. Role of long-chain acyl-coenzyme A synthetases in the regulation of arachidonic acid metabolism in interleukin 1β-stimulated rat fibroblasts. Biochim Biophys Acta 2014; 1841:44–53. doi: 10.1016/j.bbalip.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 94.Oh-ishi S, Yamaki K, Abe M, Tomoda H, Omura S. The acyl-CoA synthetase inhibitor triacsin C enhanced eicosanoid release in leukocytes. Jpn J Pharmacol 1992; 59:417–418. doi: 10.1254/jjp.59.417. [DOI] [PubMed] [Google Scholar]
- 95.Maloberti PM, Duarte AB, Orlando UD, Pasqualini ME, Solano AR, López-Otín C, et al. Functional interaction between acyl-CoA synthetase 4, lipooxygenases and cyclooxygenase-2 in the aggressive phenotype of breast cancer cells. PloS One 2010; 5:e15540.doi: 10.1371/journal.pone.0015540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011; 32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shikita M, Hall PF. The stoichiometry of the conversion of cholesterol and hydroxycholesterols to pregnenolone (3beta-hydroxypregn-5-en-20-one) catalyzed by adrenal cytochrome P-450. Proc Natl Acad Sci U S A 1974; 71:1441–1445. doi: 10.1073/pnas.71.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Galano M, Li Y, Li L, Sottas C, Papadopoulos V. Role of constitutive STAR in Leydig cells. Int J Mol Sci 2021; 22:2021.doi: 10.3390/ijms22042021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hume R, Kelly RW, Taylor PL, Boyd GS. The catalytic cycle of cytochrome P-450scc and intermediates in the conversion of cholesterol to pregnenolone. Eur J Biochem 1984; 140:583–591. doi: 10.1111/j.1432-1033.1984.tb08142.x. [DOI] [PubMed] [Google Scholar]
- 100.Manna PR, Slominski AT, King SR, Stetson CL, Stocco DM. Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: involvement of cAMP/PKA signaling. Endocrinology 2014; 155:576–591. doi: 10.1210/en.2013-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, Shen CL, Dyson MT, Yin X, Schiffer RB, Grammas P, et al. The involvement of epoxygenase metabolites of arachidonic acid in cAMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J Endocrinol 2006; 190:871–878. doi: 10.1677/joe.1.06933. [DOI] [PubMed] [Google Scholar]
- 102.Wang XJ, Dyson MT, Jo Y, Eubank DW, Stocco DM. Involvement of 5-lipoxygenase metabolites of arachidonic acid in cyclic AMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J Steroid Biochem Mol Biol 2003; 85:159–166. doi: 10.1016/s0960-0760(03)00189-4. [DOI] [PubMed] [Google Scholar]
- 103.Wang X, Walsh LP, Reinhart AJ, Stocco DM. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem 2000; 275:20204–20209. doi: 10.1074/jbc.M003113200. [DOI] [PubMed] [Google Scholar]
- 104.Cornejo Maciel F, Maloberti P, Neuman I, Cano F, Castilla R, Castillo F, et al. An arachidonic acid-preferring acyl-CoA synthetase is a hormone-dependent and obligatory protein in the signal transduction pathway of steroidogenic hormones. J Mol Endocrinol 2005; 34:655–666. doi: 10.1677/jme.1.01691. [DOI] [PubMed] [Google Scholar]
- 105.Castillo AF, Cornejo Maciel F, Castilla R, Duarte A, Maloberti P, Paz C, et al. cAMP increases mitochondrial cholesterol transport through the induction of arachidonic acid release inside this organelle in Leydig cells. FEBS J 2006; 273:5011–5021. doi: 10.1111/j.1742-4658.2006.05496.x. [DOI] [PubMed] [Google Scholar]
- 106.Maloberti P, Castilla R, Castillo F, Cornejo Maciel F, Mendez CF, Paz C, et al. Silencing the expression of mitochondrial acyl-CoA thioesterase I and acyl-CoA synthetase 4 inhibits hormone-induced steroidogenesis. FEBS J 2005; 272:1804–1814. doi: 10.1111/j.1742-4658.2005.04616.x. [DOI] [PubMed] [Google Scholar]
- 107.Dattilo MA, Benzo Y, Herrera LM, Prada JG, Lopez PF, Caruso CM, et al. Regulation and role of acyl-CoA synthetase 4 in glial cells. J Steroid Biochem Mol Biol 2021; 208:105792.doi: 10.1016/j.jsbmb.2020.105792. [DOI] [PubMed] [Google Scholar]
- 108.Cho YY, Kang MJ, Sone H, Suzuki T, Abe M, Igarashi M, et al. Abnormal uterus with polycysts, accumulation of uterine prostaglandins, and reduced fertility in mice heterozygous for acyl-CoA synthetase 4 deficiency. Biochem Biophys Res Commun 2001; 284:993–997. doi: 10.1006/bbrc.2001.5065. [DOI] [PubMed] [Google Scholar]
- 109.Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun C, et al. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun 2021; 93:312–321. doi: 10.1016/j.bbi.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 110.Drijvers JM, Gillis JE, Muijlwijk T, Nguyen TH, Gaudiano EF, Harris IS, et al. Pharmacologic screening identifies metabolic vulnerabilities of CD8+ T cells. Cancer Immunol Res 2021; 9:184–199. doi: 10.1158/2326-6066.CIR-20-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y, et al. CD8 T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022; 40:365–378.e6. doi: 10.1016/j.ccell.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ansari IH, Longacre MJ, Stoker SW, Kendrick MA, O’Neill LM, Zitur LJ, et al. Characterization of Acyl-CoA synthetase isoforms in pancreatic beta cells: gene silencing shows participation of ACSL3 and ACSL4 in insulin secretion. Arch Biochem Biophys 2017; 618:32–43. doi: 10.1016/j.abb.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kung YA, Chiang HJ, Li ML, Gong YN, Chiu HP, Hung CT, et al. Acyl-coenzyme A synthetase long-chain family member 4 is involved in viral replication organelle formation and facilitates virus replication via ferroptosis. mBio 2022; 13:e0271721.doi: 10.1128/mbio.02717-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu N, Bai L, Dai E, Han L, Kang R, Li H, et al. Pirin is a nuclear redox-sensitive modulator of autophagy-dependent ferroptosis. Biochem Biophys Res Commun 2021; 536:100–106. doi: 10.1016/j.bbrc.2020.12.066. [DOI] [PubMed] [Google Scholar]
- 115.Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep 2020; 33:108487.doi: 10.1016/j.celrep.2020.108487. [DOI] [PubMed] [Google Scholar]
- 116.Chen WC, Wang CY, Hung YH, Weng TY, Yen MC, Lai MD. Systematic analysis of gene expression alterations and clinical outcomes for long-chain acyl-coenzyme A synthetase family in cancer. PloS One 2016; 11:e0155660.doi: 10.1371/journal.pone.0155660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu Y, Sun X, Chen F, Liu M. Genetic alteration, prognostic and immunological role of acyl-CoA synthetase long-chain family member 4 in a pan-cancer analysis. Front Genet 2022; 13:812674.doi: 10.3389/fgene.2022.812674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen J, Ding C, Chen Y, Hu W, Lu Y, Wu W, et al. ACSL4 promotes hepatocellular carcinoma progression via c-Myc stability mediated by ERK/FBW7/c-Myc axis. Oncogenesis 2020; 9:42.doi: 10.1038/s41389-020-0226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ndiaye H, Liu JY, Hall A, Minogue S, Morgan MY, Waugh MG. Immunohistochemical staining reveals differential expression of ACSL3 and ACSL4 in hepatocellular carcinoma and hepatic gastrointestinal metastases. Biosci Rep 2020; 40:BSR20200219.doi: 10.1042/BSR20200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun XJ, Xu GL. Overexpression of acyl-CoA ligase 4 (ACSL4) in patients with hepatocellular carcinoma and its prognosis. Med Sci Monit 2017; 23:4343–4350. doi: 10.12659/msm.906639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H, Song J, He Y, Liu Y, Liu Z, Sun W, et al. CRISPR/Cas9 screens reveal that hexokinase 2 enhances cancer stemness and tumorigenicity by activating the ACSL4-fatty acid β-oxidation pathway. Adv Sci (Weinh) 2022; 9:e2105126.doi: 10.1002/advs.202105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen J, Ding C, Chen Y, Hu W, Yu C, Peng C, et al. ACSL4 reprograms fatty acid metabolism in hepatocellular carcinoma via c-Myc/SREBP1 pathway. Cancer Lett 2021; 502:154–165. doi: 10.1016/j.canlet.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 123.Wang J, Wang Z, Yuan J, Wang J, Shen X. The positive feedback between ACSL4 expression and O-GlcNAcylation contributes to the growth and survival of hepatocellular carcinoma. Aging (Albany NY) 2020; 12:7786–7800. doi: 10.18632/aging.103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev 2012; 249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu CL, Zhao SP, Yu BL. Microarray analysis provides new insights into the function of apolipoprotein O in HepG2 cell line. Lipids Health Dis 2013; 12:186.doi: 10.1186/1476-511X-12-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Du X, Zhang Y. Integrated analysis of immunity- and ferroptosis-related biomarker signatures to improve the prognosis prediction of hepatocellular carcinoma. Front Genet 2020; 11:614888.doi: 10.3389/fgene.2020.614888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feng J, Lu PZ, Zhu GZ, Hooi SC, Wu Y, Huang XW, et al. ACSL4 is a predictive biomarker of sorafenib sensitivity in hepatocellular carcinoma. Acta Pharmacol Sin 2021; 42:160–170. doi: 10.1038/s41401-020-0439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xia H, Lee KW, Chen J, Kong SN, Sekar K, Deivasigamani A, et al. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death Discov 2017; 3:17058.doi: 10.1038/cddiscovery.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Monaco ME, Creighton CJ, Lee P, Zou X, Topham MK, Stafforini DM. Expression of long-chain fatty acyl-CoA synthetase 4 in breast and prostate cancers is associated with sex steroid hormone receptor negativity. Transl Oncol 2010; 3:91–98. doi: 10.1593/tlo.09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yen MC, Kan JY, Hsieh CJ, Kuo PL, Hou MF, Hsu YL. Association of long-chain acyl-coenzyme A synthetase 5 expression in human breast cancer by estrogen receptor status and its clinical significance. Oncol Rep 2017; 37:3253–3260. doi: 10.3892/or.2017.5610. [DOI] [PubMed] [Google Scholar]
- 131.Sha R, Xu Y, Yuan C, Sheng X, Wu Z, Peng J, et al. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. EbioMedicine 2021; 71:103560.doi: 10.1016/j.ebiom.2021.103560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu X, Li Y, Wang J, Wen X, Marcus MT, Daniels G, et al. Long chain fatty acyl-CoA synthetase 4 is a biomarker for and mediator of hormone resistance in human breast cancer. PloS One 2013; 8:e77060.doi: 10.1371/journal.pone.0077060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dattilo MA, Benzo Y, Herrera LM, Prada JG, Castillo AF, Orlando UD, et al. Regulatory mechanisms leading to differential acyl-CoA synthetase 4 expression in breast cancer cells. Sci Rep 2019; 9:10324.doi: 10.1038/s41598-019-46776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H, Xu B, Zhang X, Zheng Y, Zhao Y, Chang X. PADI2 gene confers susceptibility to breast cancer and plays tumorigenic role via ACSL4, BINC3 and CA9 signaling. Cancer Cell Int 2016; 16:61.doi: 10.1186/s12935-016-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Belkaid A, Ouellette RJ, Surette ME. 17β-estradiol-induced ACSL4 protein expression promotes an invasive phenotype in estrogen receptor positive mammary carcinoma cells. Carcinogenesis 2017; 38:402–410. doi: 10.1093/carcin/bgx020. [DOI] [PubMed] [Google Scholar]
- 136.Orlando UD, Castillo AF, Medrano MAR, Solano AR, Maloberti PM, Podesta EJ. Acyl-CoA synthetase-4 is implicated in drug resistance in breast cancer cell lines involving the regulation of energy-dependent transporter expression. Biochem Pharmacol 2019; 159:52–63. doi: 10.1016/j.bcp.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 137.Kwon YS, Lee MG, Baek J, Kim NY, Jang H, Kim S. Acyl-CoA synthetase-4 mediates radioresistance of breast cancer cells by regulating FOXM1. Biochem Pharmacol 2021; 192:114718.doi: 10.1016/j.bcp.2021.114718. [DOI] [PubMed] [Google Scholar]
- 138.Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res 2020; 30:146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu X, Deng F, Li Y, Daniels G, Du X, Ren Q, et al. ACSL4 promotes prostate cancer growth, invasion and hormonal resistance. Oncotarget 2015; 6:44849–44863. doi: 10.18632/oncotarget.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma Y, Zhang X, Alsaidan OA, Yang X, Sulejmani E, Zha J, et al. Long-chain acyl-CoA synthetase 4-mediated fatty acid metabolism sustains androgen receptor pathway-independent prostate cancer. Mol Cancer Res 2021; 19:124–135. doi: 10.1158/1541-7786.MCR-20-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang X, Guo S, Zhang Y, Zhao Y, Li X, Jia Y, et al. LncRNA NEAT1 promotes docetaxel resistance in prostate cancer by regulating ACSL4 via sponging miR-34a-5p and miR-204-5p. Cell Signal 2020; 65:109422.doi: 10.1016/j.cellsig.2019.109422. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, Li S, Li F, Lv C, Yang QK. High-fat diet impairs ferroptosis and promotes cancer invasiveness via downregulating tumor suppressor ACSL4 in lung adenocarcinoma. Biol Direct 2021; 16:10.doi: 10.1186/s13062-021-00294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu H, Liu A. Long non-coding RNA NEAT1 regulates ferroptosis sensitivity in non-small-cell lung cancer. J Int Med Res 2021; 49:300060521996183.doi: 10.1177/0300060521996183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu KT, Yeh IJ, Chou SK, Yen MC, Kuo PL. Regulatory mechanism of fatty acid-CoA metabolic enzymes under endoplasmic reticulum stress in lung cancer. Oncol Rep 2018; 40:2674–2682. doi: 10.3892/or.2018.6664. [DOI] [PubMed] [Google Scholar]
- 145.Tang X, Ding H, Liang M, Chen X, Yan Y, Wan N, et al. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thoracic cancer 2021; 12:1219–1230. doi: 10.1111/1759-7714.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bach DH, Luu TT, Kim D, An YJ, Park S, Park HJ, et al. BMP4 upregulation is associated with acquired drug resistance and fatty acid metabolism in EGFR-mutant non-small-cell lung cancer cells. Mol Ther Nucleic Acids 2018; 12:817–828. doi: 10.1016/j.omtn.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ye S, Xu M, Zhu T, Chen J, Shi S, Jiang H, et al. Cytoglobin promotes sensitivity to ferroptosis by regulating p53-YAP1 axis in colon cancer cells. J Cell Mol Med 2021; 25:3300–3311. doi: 10.1111/jcmm.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tian X, Li S, Ge G. Apatinib promotes ferroptosis in colorectal cancer cells by targeting ELOVL6/ACSL4 signaling. Cancer Manag Res 2021; 13:1333–1342. doi: 10.2147/CMAR.S274631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park S, Oh J, Kim M, Jin EJ. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim Cells Syst (Seoul) 2018; 22:334–340. doi: 10.1080/19768354.2018.1512521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng J, Fan YQ, Liu BH, Zhou H, Wang JM, Chen QX. ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol Rep 2020; 43:147–158. doi: 10.3892/or.2019.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou L, Wang Z, Hu C, Zhang C, Kovatcheva-Datchary P, Yu D, et al. Integrated metabolomics and lipidomics analyses reveal metabolic reprogramming in human glioma with IDH1 mutation. J Proteome Res 2019; 18:960–969. doi: 10.1021/acs.jproteome.8b00663. [DOI] [PubMed] [Google Scholar]
- 152.Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nature Commun 2020; 11:5424.doi: 10.1038/s41467-020-19193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tan S, Hou X, Mei L. Dihydrotanshinone I inhibits human glioma cell proliferation via the activation of ferroptosis. Oncol Lett 2020; 20:122.doi: 10.3892/ol.2020.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yi R, Wang H, Deng C, Wang X, Yao L, Niu W, et al. Dihydroartemisinin initiates ferroptosis in glioblastoma through GPX4 inhibition. Biosci Rep 2020; 40:BSR20193314.doi: 10.1042/BSR20193314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bai R, Rebelo A, Kleeff J, Sunami Y. Identification of prognostic lipid droplet-associated genes in pancreatic cancer patients via bioinformatics analysis. Lipids Health Dis 2021; 20:58.doi: 10.1186/s12944-021-01476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ye Z, Hu Q, Zhuo Q, Zhu Y, Fan G, Liu M, et al. Abrogation of ARF6 promotes RSL3-induced ferroptosis and mitigates gemcitabine resistance in pancreatic cancer cells. Am J Cancer Res 2020; 10:1182–1193. [PMC free article] [PubMed] [Google Scholar]
- 157.Huang XD, Xiao FJ, Guo YT, Sun Y, Zhang YK, Shi XJ. Protein tyrosine phosphatase 1 protects human pancreatic cancer from erastin-induced ferroptosis. Asian J Surg 2022; 45:2214–2223. doi: 10.1016/j.asjsur.2021.11.048. [DOI] [PubMed] [Google Scholar]
- 158.Ou R, Lu S, Wang L, Wang Y, Lv M, Li T, et al. Circular RNA circLMO1 suppresses cervical cancer growth and metastasis by triggering miR-4291/-mediated ferroptosis. Front Oncol 2022; 12:858598.doi: 10.3389/fonc.2022.858598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xiaofei J, Mingqing S, Miao S, Yizhen Y, Shuang Z, Qinhua X, et al. Oleanolic acid inhibits cervical cancer Hela cell proliferation through modulation of the ACSL4 ferroptosis signaling pathway. Biochem Biophys Res Commun 2021; 545:81–88. doi: 10.1016/j.bbrc.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 160.Tuo QZ, Liu Y, Xiang Z, Yan HF, Zou T, Shu Y, et al. Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct Target Ther 2022; 7:59.doi: 10.1038/s41392-022-00917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun W, Wu X, Yu P, Zhang Q, Shen L, Chen J, et al. LncAABR07025387.1 enhances myocardial ischemia/reperfusion injury miR-205/ACSL4-mediated ferroptosis. Front Cell Dev Biol 2022; 10:672391.doi: 10.3389/fcell.2022.672391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fan Z, Cai L, Wang S, Wang J, Chen B. Baicalin prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis. Front Pharmacol 2021; 12:628988.doi: 10.3389/fphar.2021.628988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu Y, Li X, Cheng Y, Yang M, Wang R. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB J 2020; 34:16262–16275. doi: 10.1096/fj.202001758R. [DOI] [PubMed] [Google Scholar]
- 164.Wang Y, Bi R, Quan F, Cao Q, Lin Y, Yue C, et al. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur J Pharmacol 2020; 888:173574.doi: 10.1016/j.ejphar.2020.173574. [DOI] [PubMed] [Google Scholar]
- 165.Liu C, Sun W, Zhu T, Shi S, Zhang J, Wang J, et al. Glia maturation factor-β induces ferroptosis by impairing chaperone-mediated autophagic degradation of ACSL4 in early diabetic retinopathy. Redox Biology 2022; 52:102292.doi: 10.1016/j.redox.2022.102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu Y, Zhao Y, Yang HZ, Wang YJ, Chen Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci Rep 2021; 41:BSR20202924.doi: 10.1042/BSR20202924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang XJ, Zhou L, Lu WJ, Du WX, Mi XY, Li Z, et al. Comparative transcriptomic analysis reveals an association of gibel carp fatty liver with ferroptosis pathway. BMC Genomics 2021; 22:328.doi: 10.1186/s12864-021-07621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wei S, Qiu T, Wang N, Yao X, Jiang L, Jia X, et al. Ferroptosis mediated by the interaction between Mfn2 and IREα promotes arsenic-induced nonalcoholic steatohepatitis. Environ Res 2020; 188:109824.doi: 10.1016/j.envres.2020.109824. [DOI] [PubMed] [Google Scholar]
- 169.Zhang RN, Pan Q, Zheng RD, Mi YQ, Shen F, Zhou D, et al. Genome-wide analysis of DNA methylation in human peripheral leukocytes identifies potential biomarkers of nonalcoholic fatty liver disease. Int J Mol Med 2018; 42:443–452. doi: 10.3892/ijmm.2018.3583. [DOI] [PubMed] [Google Scholar]
- 170.Zhou Y, Zhou H, Hua L, Hou C, Jia Q, Chen J, et al. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic Biol Med 2021; 171:55–68. doi: 10.1016/j.freeradbiomed.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 171.Shao G. Integrated RNA gene expression analysis identified potential immune-related biomarkers and RNA regulatory pathways of acute myocardial infarction. PloS One 2022; 17:e0264362.doi: 10.1371/journal.pone.0264362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rossi Sebastiano M, Konstantinidou G. Targeting long chain acyl-CoA synthetases for cancer therapy. Int J Mol Sci 2019; 20:3624.doi: 10.3390/ijms20153624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Taylor WR, Fedorka SR, Gad I, Shah R, Alqahtani HD, Koranne R, et al. Small-molecule ferroptotic agents with potential to selectively target cancer stem cells. Sci Rep 2019; 9:5926.doi: 10.1038/s41598-019-42251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cotte AK, Aires V, Fredon M, Limagne E, Derangère V, Thibaudin M, et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat Commun 2018; 9:322.doi: 10.1038/s41467-017-02732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]