Abstract
BACKGROUND
Astroblastoma is a rare neoplasm characterized as a circumscribed glial neoplasm most often arising in the frontoparietal cerebral hemispheres in older children.
OBSERVATIONS
We report an intriguing case of an astroblastoma recurrence 21 years after gross-total resection and radiation. A 32-year-old right-handed female presented to the emergency department for a generalized tonic-clonic seizure. She had a history of bipolar disorder, intractable migraines, and prior seizures linked to an astroblastoma previously resected three times. Magnetic resonance imaging on the current visit showed growth of the recurrent lesion to a 3.8-cm maximal diameter. Left-sided awake craniotomy was performed to remove the tumor while using speech mapping and 5-aminolevulinic acid (5-ALA). Targeted next-generation sequencing of the tumor revealed in-frame MN1::BEND2 fusion transcripts.
LESSONS
We found that 5-ALA can be used in astroblastoma patients to assist in gross-total resection, which is important for long-term survival. Our astroblastoma case demonstrated classic astroblastoma morphology, with typical perivascular astroblastic rosettes, and was brightly fluorescent after 5-ALA administration.
Keywords: astroblastoma, 5-ALA, awake resection, recurrent astroblastoma, 5-aminolevulinic acid
ABBREVIATIONS: 5-ALA = 5-aminolevulinic acid, MRI = magnetic resonance imaging, WHO = World Health Organization
Astroblastoma is a rare, circumscribed glial neoplasm most often arising in the frontoparietal cerebral hemispheres in older children and young adults.1–4 Astroblastomas are typically cortically based but occasionally extend to the subarachnoid spaces. Other less common locations include the hypothalamus, brainstem, intraventricular space, and cerebellum.3 Characteristic histology overlaps somewhat with ependymomas and includes vascular hyalinization as well as astroblastic pseudorosettes, which demonstrate GFAP-positive glial cells with perivascular broad process arrangements.5 First reported by Bailey and Cushing in 1926,6 this tumor historically had an ambiguous definition with much dispute about its existence. Astroblastomas reportedly account for 0.45% to 2.8% of all primary glial tumors.7 The rate of misdiagnosis of these tumors is high because of its rarity and its similarity to other glial neoplasms.8 Common clinical symptoms are nonspecific and include headache, seizures, nausea, and focal neurological deficits.9,10 We report an intriguing case of astroblastoma recurrence 21 years after gross-total resection and radiation.
Illustrative Case
A 32-year-old right-handed female presented to the emergency department for a generalized tonic-clonic seizure. She had a history of bipolar disorder, intractable migraines, and prior seizures related to a previously resected astroblastoma. She underwent initial resection at age 5 (Fig. 1A and B). At age 11, she had regrowth of the tumor and underwent repeat resection followed by radiation (Fig. 1C and D). She had one emergency visit 4 years prior to her current presentation because of a seizure, although this was her first seizure since her last surgery. Magnetic resonance imaging (MRI) on that visit demonstrated a stable 6 mm × 6 mm nodular enhancing focus in the inferolateral aspect of the resection cavity that had not changed in size compared to prior follow-up scans.
FIG. 1.
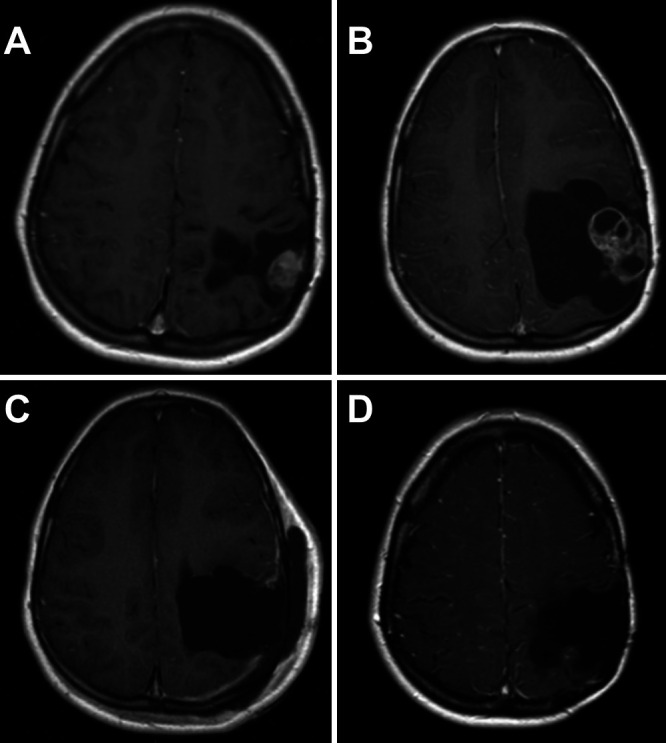
MRI studies from childhood. A: Residual focus of nodular enhancement on postoperative MRI after the first resection at age 5. B: There is a significant increase in the residual enhancing lesion on the preoperative MRI prior to her second resection at age 5. C: Postoperative imaging demonstrates gross-total resection with no residual enhancing tumor after surgery at age 11. D: Eight-year follow-up MRI after her third resection and adjuvant radiation, displaying no tumor regrowth at age 19.
MRI on the current visit showed significant regrowth to 3.8 cm. The tumor was lobulated, T1 and T2 isointense, and with heterogeneous contrast enhancement at the anterior inferior aspect of the resection cavity with a component of mass effect on the left lateral ventricle atrium without midline shift (Fig. 2). A left-sided awake craniotomy was performed (Video 1) to remove the tumor while using speech mapping and 5-aminolevulinic acid (5-ALA). Speech mapping of the resection area showed no response to direct cortical stimulation. The tumor fluoresced heterogeneously, with some areas of very bright fluorescence (Fig. 3). At the completion of resection, no further fluorescence was present in the margins of the resection cavity. Postoperative MRI showed a 1-cm area of contrast enhancement along the tumor bed. The patient returned to the operating room 2 days later, and this region was sampled with frozen section pathology, demonstrating normal brain. She was discharged 2 days later at her neurological baseline. Three-month postoperative MRI confirmed gross-total resection (Fig. 4).
FIG. 2.
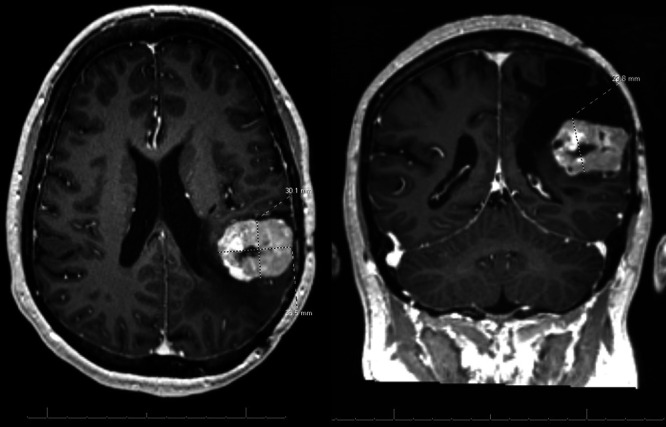
Preoperative MRI at age 32 demonstrates recurrent, strongly enhancing tumor at the anterior inferior aspect of the left parietal resection cavity, measuring 3.8 × 2.9 × 3.0 cm.
FIG. 3.
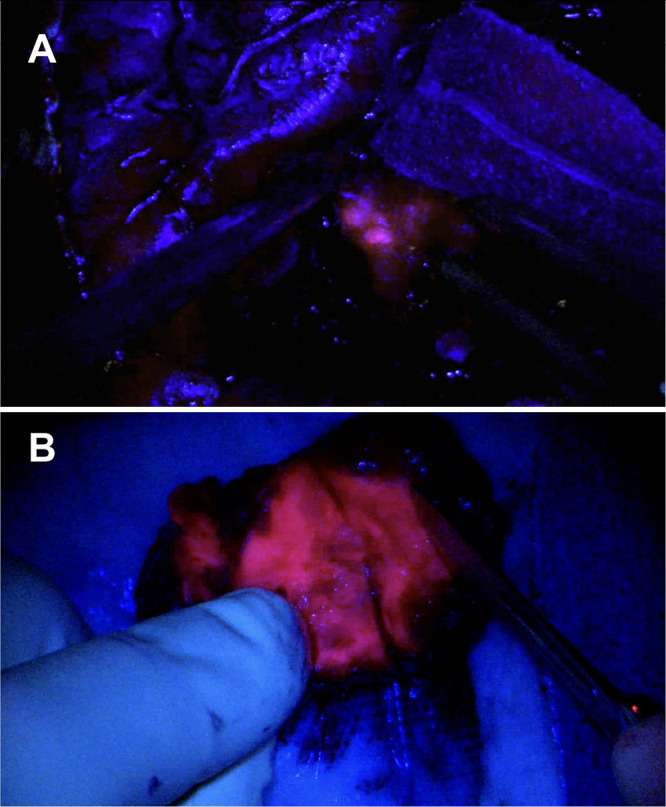
A: Fluorescence noted intraoperatively under blue light after 5-ALA administration. B: The completely resected tumor showed bright heterogeneous fluorescence.
FIG. 4.
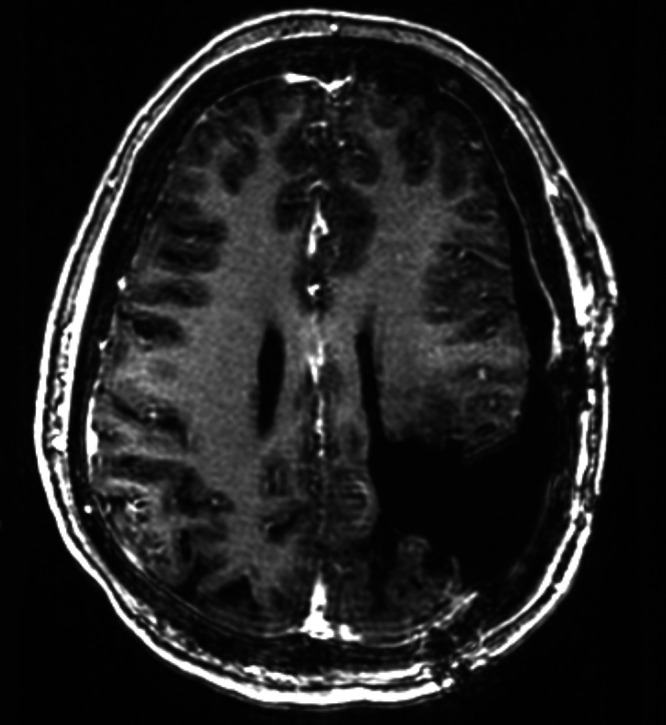
Postoperative day 1 MRI after her most recent resection (age 32) confirms gross-total resection of the large recurrent tumor.
VIDEO 1. Clip showing evidence of the growing benefits of using 5-ALA in astroblastoma patients to assist in gross-total resection, which is paramount for long-term survival. We present a compelling case of astroblastoma recurrence 21 years after gross-total resection and radiation. A 32-year-old female presented to the emergency department for a generalized tonic-clonic seizure linked to an astroblastoma, previously resected three times. This video demonstrates the benefit of using 5-ALA for successful gross-total resection of an astroblastoma. Click here to view.
Histology
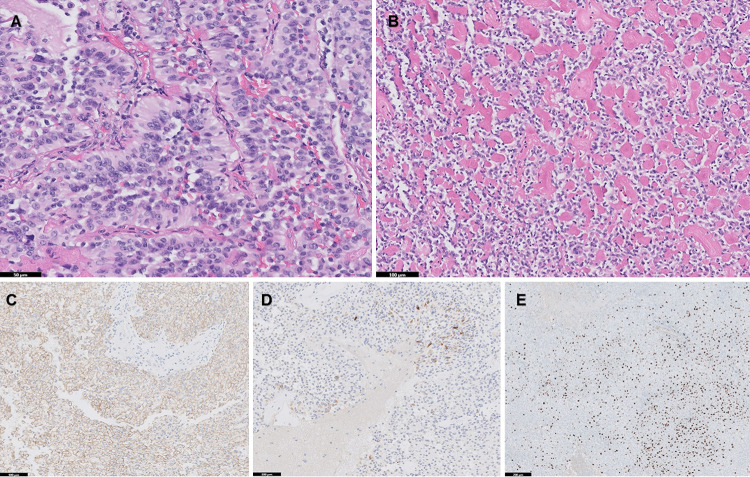
Histological analysis demonstrated classic astroblastoma morphology, with typical perivascular astroblastic rosettes composed of stout cytoplasmic processes radiating around thin-walled vessels (Fig. 5A). Many areas of the tumor demonstrated perivascular and stromal sclerosis (Fig. 5B). Mitotic activity was brisk in some areas, with focally up to 17 mitoses/10 high-power fields and microscopic foci of necrosis. On immunohistochemical analysis, the tumor showed diffuse membranous EMA expression (Fig. 5C), with GFAP (Fig. 5D) expression only in a minority of cells. The Ki-67 was high, with up to 25% to 30% proliferation index in the area of highest labeling (Fig. 5E). Targeted next-generation sequencing of the tumor demonstrated an in-frame MN1::BEND2 fusion and was negative for BRAF p.V600E mutation.
FIG. 5.
Histological features of astroblastoma. A: Well-developed perivascular astroblastic pseudorosettes with stout cytoplasmic processes radiating around the vessel walls (hematoxylin and eosin [H&E], Bar = 50 µm). B: Prominent perivascular and stromal sclerosis, typical of astroblastoma (H&E, Bar = 100 µm). C: Diffuse membranous reactivity for EMA (Bar = 100 µm). D: GFAP expression seen in the minority of cells (Bar = 100 µm). E: Ki-67 demonstrates 25% to 30% proliferation index in the area of highest labeling (Bar = 200 µm).
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
This case is notable for two primary factors. First, it exemplifies late recurrence 20 years after gross-total resection and radiation treatment. Seizures were the patient’s only symptom of the recurrence. Second, this is the third case, to our knowledge, of successfully utilizing 5-ALA fluorescence to aid in resection. Ahmed et al.11 reported the largest series of patients with 239 identified astroblastomas, 209 of which received treatment. Astroblastomas have been more frequently described in females.12,13 The literature supports astroblastoma occurrence in a bimodal distribution, with a peak between the ages of 5 and 10 years old and another between the ages of 21 and 30 years old.14
Lessons
Since the initial description of astroblastomas by Bailey and Cushing,6 seemingly the only agreed-upon point in the literature is the lack of consensus on the diagnoses, classifications, and histogenesis of these tumors.7,15,16 Some characteristically observed features include the perivascular origination of neoplastic cells that stain positive for GFAP, vimentin, and S-100 protein.17 Astroblastomas can often be mistaken for ependymomas and other infiltrating gliomas.18 However, ependymomas have fine fibrillary cell processes as opposed to the stout columnar processes typical of astroblastoma.18 Recurrent rearrangements of the MN1 gene have recently been described in astroblastomas; these alterations at chromosome 22q12.1 most frequently occur in-frame with BEND2 at Xp22.13, but other fusion partners have been described.1 These diagnostic alterations can be detected by fluorescence in-situ hybridization, reverse transcriptase-polymerase chain reaction, or next-generation RNA or DNA sequencing. Additionally, a series of 28 cases of astroblastoma revealed BRAF V600E mutations in 38% of cases, a potentially targetable mutation.5 The most recent World Health Organization (WHO) classification of central nervous system tumors (2021) recognizes “astroblastoma, MN1-altered” as a distinct integrated molecular and histological diagnosis. In the absence of confirmatory molecular testing, such tumors are histologically classified as “astroblastoma, not otherwise specified.”12
Because of the rarity of this tumor, a definitive WHO grading system has not been established for astroblastoma. However, high-grade histology has been found to be associated with recurrence, tumor progression, and a worse prognosis in the limited studies of histologically defined astroblastomas.10 Although the WHO does not recognize a grading system, others have described “low-grade” and “high-grade” astroblastomas.4 Low-grade astroblastomas have been described as having orderly perivascular pseudorosettes, a low mitotic index, minimal cellular atypia, little or no proliferation of the vascular endothelium, and sclerosis of the vessel walls.4 High-grade astroblastomas have frequent cytological atypia, multiple layers of compact perivascular cells, a high mitotic index, hypertrophy and hyperplasia of the vascular endothelium, and minimal sclerosis of the vessel walls.4 The tumor in our patient had features of both high- and low-grade astroblastoma according to this description. However, scientific dogma may have exceptions in astroblastomas; hence, there is no grading criteria for these tumors. There have been reports of early recurrences in low-grade astroblastomas as well as multiple cases of high-grade astroblastomas with high long-term survival outcomes.19–23
The most important factor in survival outcomes in astroblastoma is the extent of resection.16 The differences between total and subtotal resections are drastic, and the 5-year-survival rate difference has been reported as 83% in total resection versus 55% in subtotal resection.24 Adjuvant therapy has been attempted in multiple studies with mixed results, with no study able to statistically demonstrate improved survival outcomes from radiation or chemotherapy.4,12,19 Thus, the achievement of gross-total resection should have overriding importance in the future treatment of these tumors.
5-ALA is a precursor compound involved in heme synthesis that is converted to the fluorogenic metabolite protoporphyrin IX. Certain tumors consume 5-ALA at higher rates than surrounding tissue, allowing for tumor-specific fluorescence under blue light that aids in gross-total resection, which improves progression-free survival rates.25 Given the large body of literature supporting 5-ALA fluorescence in gliomas, particularly glioblastoma multiforme, 5-ALA was utilized to aid in the resection of the recurrent tumor in our patient.26 5-ALA is well documented to improve the extent of resection and progression-free survival in high-grade gliomas.27–29 5-ALA has sometimes shown fluorescence in low-grade gliomas as well, albeit at lower rates and lower, less substantiated efficacy levels.30
A PubMed search for (“astroblastoma”) and (5-ALA) yielded only two results of previously reported cases of 5-ALA–induced fluorescence of astroblastomas. One case in a 13-year-old female and another in a 6-year-old female resulted in total tumor removal after fluorescence-guided resection.14,31 Fluorescence was reported even in the case in which the tumor was described as low-grade histologically by Agawa et al.,31 as well as by Fudaba et al.,14 who did not give a histological grade but did describe an MN1 alteration. A limitation of using 5-ALA in astroblastoma is that the more encapsulated and less dispersive nature of these lesions lessens the potential need for 5-ALA in astroblastoma cases compared to high-grade gliomas since more compact lesions are easier for surgeons to visualize and resect. Because astroblastomas are so rare, it is also highly unlikely that there will ever be enough cases to warrant larger trials with 5-ALA in line with the high-grade glioma trials. However, total resection of astroblastoma has shown significantly improved patient outcomes compared to subtotal resection, indicating that the added benefit of using 5-ALA to aid in gross-total resection may be warranted.24
A notable aspect for surgeons desiring to use 5-ALA involves educating the care team and the patient that lights in the operating room and the patient’s postoperative hospital room should be kept dim in the 24 hours postadministration because of the extremely rare but reported incidents of phototoxicity.32 This challenge can be intensified by the on-and-off nature of the overhead lights in the operating room during awake craniotomies. Extra care must be taken to ensure that as much of the patient’s exposed skin surfaces as possible are shaded while the patient can still maintain visualization of the speech pathologist or team member in charge of communication during the awake period of the surgery.
We utilized 5-ALA in our case to obtain the most complete resection and thus the best outcome possible given her multiple recurrences. The majority of the tumor fluoresced brightly, though some regions did not. These findings demonstrate that 5-ALA can aid in achieving gross-total resection for astroblastoma. Astroblastomas are exceptionally uncommon glial tumors that typically present bimodally in children and young adults in supratentorial locations, frequently in the frontoparietal cerebral hemispheres. Our case highlighted the potential for long-term recurrence, even decades after gross-total resection and radiation. In addition, strong tumor fluorescence following 5-ALA administration highlights a potential adjunct technique that may aid in the resection of these tumors.
Author Contributions
Conception and design: Karas, Price. Acquisition of data: Karas, Price, O’Leary, D’Souza. Analysis and interpretation of data: Karas, Price, O’Leary, Malkova, D’Souza, Felicella. Drafting the article: Price, O’Leary, Malkova. Critically revising the article: Karas, Price, O’Leary, D’Souza, Felicella. Reviewed submitted version of manuscript: Karas, Price, O’Leary, Ogasawara, Felicella. Approved the final version of the manuscript on behalf of all authors: Karas. Administrative/technical/material support: Karas. Study supervision: Karas, Price.
Supplemental Information
Videos
Video 1. https://vimeo.com/870693307
References
- 1.Brat D, Aldape K, Idbaih A, et al. Astroblastoma, MN1-altered. WHO Classification of Tumours Editorial Board. Central nervous system tumours. In: WHO classification of tumours series, 5th ed.; vol. 6. International Agency for Research on Cancer; 2021. Accessed August 1, 2023. https://publications.iarc.fr/601.
- 2. Hirose T, Nobusawa S, Sugiyama K, et al. Astroblastoma: a distinct tumor entity characterized by alterations of the X chromosome and MN1 rearrangement. Brain Pathol. 2018;28(5):684–694. doi: 10.1111/bpa.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhalerao S, Nagarkar R, Adhav A. A case report of high-grade astroblastoma in a young adult. CNS Oncol. 2019;8(1):CNS29. doi: 10.2217/cns-2018-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonnin JMMD, Rubinstein LJMD. Astroblastomas: a pathological study of 23 tumors, with a postoperative follow-up in 13 patients. Neurosurgery. 1989;25(1):6–13. [PubMed] [Google Scholar]
- 5. Lehman NL, Hattab EM, Mobley BC, et al. Morphological and molecular features of astroblastoma, including BRAFV600E mutations, suggest an ontological relationship to other cortical-based gliomas of children and young adults. Neuro Oncol. 2017;19(1):31–42. doi: 10.1093/neuonc/now118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey P, Cushing H. A classification of tumors of the glioma group on a histogenetic basis with a correlation study of prognosis. Lippincott; 1926. [Google Scholar]
- 7. Khosla D, Yadav BS, Kumar R, et al. Pediatric astroblastoma: a rare case with a review of the literature. Pediatr Neurosurg. 2012;48(2):122–125. doi: 10.1159/000342538. [DOI] [PubMed] [Google Scholar]
- 8. Ramesh S, Raju S. Pediatric astroblastoma: case report and review of the literature. Indian J Neurosurg. 2014;3(3):174–174. [Google Scholar]
- 9. Sadiq M, Ahmad I, Shuja J, Ahmad Z, Ahmed R, Ahmad K. Astroblastoma in a young female patient: a case report and literature review of clinicopathological, radiological and prognostic characteristics and current treatment strategies. Brain Tumor Res Treat. 2017;5(2):120–126. doi: 10.14791/btrt.2017.5.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen F, Chen LC, Yao Y, Zhou LF. Astroblastoma: rare incidence and challenges in the pattern of care. World Neurosurg. 2014;82(1-2):e125–e127. doi: 10.1016/j.wneu.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed KA, Allen PK, Mahajan A, Brown PD, Ghia AJ. Astroblastomas: a Surveillance, Epidemiology, and End Results (SEER)-based patterns of care analysis. World Neurosurg. 2014;82(1-2):e291–e297. doi: 10.1016/j.wneu.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 12. Tumialán LMMD, Brat DJMD, Fountain AJMD, Barrow DLMD. An astroblastoma mimicking a cavernous malformation: case report. Neurosurgery. 2007;60(3):E569–E570. doi: 10.1227/01.NEU.0000255336.80285.70. [DOI] [PubMed] [Google Scholar]
- 13. Bell JW, Osborn AG, Salzman KL, Blaser SI, Jones BV, Chin SS. Neuroradiologic characteristics of astroblastoma. Neuroradiology. 2007;49(3):203–209. doi: 10.1007/s00234-006-0182-0. [DOI] [PubMed] [Google Scholar]
- 14. Fudaba H, Momii Y, Kawasaki Y, Goto H, Nobusawa S, Fujiki M. Well-differentiated astroblastoma with both focal anaplastic features and a meningioma 1 gene alteration. NMC Case Rep J. 2020;7(4):205–210. doi: 10.2176/nmccrj.cr.2020-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammas N, Senhaji N, Alaoui Lamrani MY, et al. Astroblastoma—a rare and challenging tumor: a case report and review of the literature. J Med Case Reports. 2018;12(1):102. doi: 10.1186/s13256-018-1623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janz C, Buhl R. Astroblastoma: report of two cases with unexpected clinical behavior and review of the literature. Clin Neurol Neurosurg. 2014;125:114–124. doi: 10.1016/j.clineuro.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 17. Denaro L, Gardiman M, Calderone M, et al. Intraventricular astroblastoma. Case report. J Neurosurg Pediatr. 2008;1(2):152–155. doi: 10.3171/PED/2008/1/2/152. [DOI] [PubMed] [Google Scholar]
- 18. Tumors of the Central Nervous System. (AFIP Atlas of Tumor Pathology, Series 4. Fascicle 7) Neuropathol Appl Neurobiol. 2008;34(4):473–474. [Google Scholar]
- 19. Thiessen B, Finlay J, Kulkarni R, Rosenblum MK. Astroblastoma: does histology predict biologic behavior? J Neurooncol. 1998;40(1):59–65. doi: 10.1023/a:1006025000409. [DOI] [PubMed] [Google Scholar]
- 20. Kaji M, Takeshima H, Nakazato Y, Kuratsu J. Low-grade astroblastoma recurring with extensive invasion. Neurol Med Chir (Tokyo) 2006;46(9):450–454. doi: 10.2176/nmc.46.450. [DOI] [PubMed] [Google Scholar]
- 21. Lau PPL, Thomas TMM, Lui PCW, Khin AT. ‘Low-grade’ astroblastoma with rapid recurrence: a case report. Pathology. 2006;38(1):78–80. doi: 10.1080/00313020500468871. [DOI] [PubMed] [Google Scholar]
- 22. Nasit JG, Trivedi P. Recurrent low-grade astroblastoma with signet ring-like cells and high proliferative index. Fetal Pediatr Pathol. 2013;32(4):284–292. doi: 10.3109/15513815.2012.754525. [DOI] [PubMed] [Google Scholar]
- 23. Miranda P, Lobato RD, Cabello A, Gómez PA, Martínez de Aragón A. Complete surgical resection of high-grade astroblastoma with long time survival: case report and review of the literature. Neurocirugia (Astur) 2006;17(1):60–63. doi: 10.1016/s1130-1473(06)70371-2. [DOI] [PubMed] [Google Scholar]
- 24. Sughrue ME, Choi J, Rutkowski MJ, et al. Clinical features and post-surgical outcome of patients with astroblastoma. J Clin Neurosci. 2011;18(6):750–754. doi: 10.1016/j.jocn.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 25. Traylor JI, Pernik MN, Sternisha AC, McBrayer SK, Abdullah KG. Molecular and metabolic mechanisms underlying selective 5-aminolevulinic acid-induced fluorescence in gliomas. Cancers (Basel) 2021;13(3):580. doi: 10.3390/cancers13030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stepp H, Stummer W. 5-ALA in the management of malignant glioma. Lasers Surg Med. 2018;50(5):399–419. doi: 10.1002/lsm.22933. [DOI] [PubMed] [Google Scholar]
- 27. Hadjipanayis CG, Stummer W. 5-ALA and FDA approval for glioma surgery. J Neurooncol. 2019;141(3):479–486. doi: 10.1007/s11060-019-03098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahmoudi K, Garvey KL, Bouras A, et al. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J Neurooncol. 2019;141(3):595–607. doi: 10.1007/s11060-019-03103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Delgado-López PD, Corrales-García EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 30. Kiesel B, Freund J, Reichert D, et al. 5-ALA in suspected low-grade gliomas: current role, limitations, and new approaches. Front Oncol. 2021;11:699301. doi: 10.3389/fonc.2021.699301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agawa Y, Wataya T. The use of 5-aminolevulinic acid to assist gross total resection of pediatric astroblastoma. Childs Nerv Syst. 2018;34(5):971–975. doi: 10.1007/s00381-017-3714-5. [DOI] [PubMed] [Google Scholar]
- 32. Yahanda AT, Dunn GP, Chicoine MR. Photosensitivity reaction from operating room lights after oral administration of 5-aminolevulinic acid for fluorescence-guided resection of a malignant glioma. Cureus. 2021;13(2):e13442. doi: 10.7759/cureus.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]