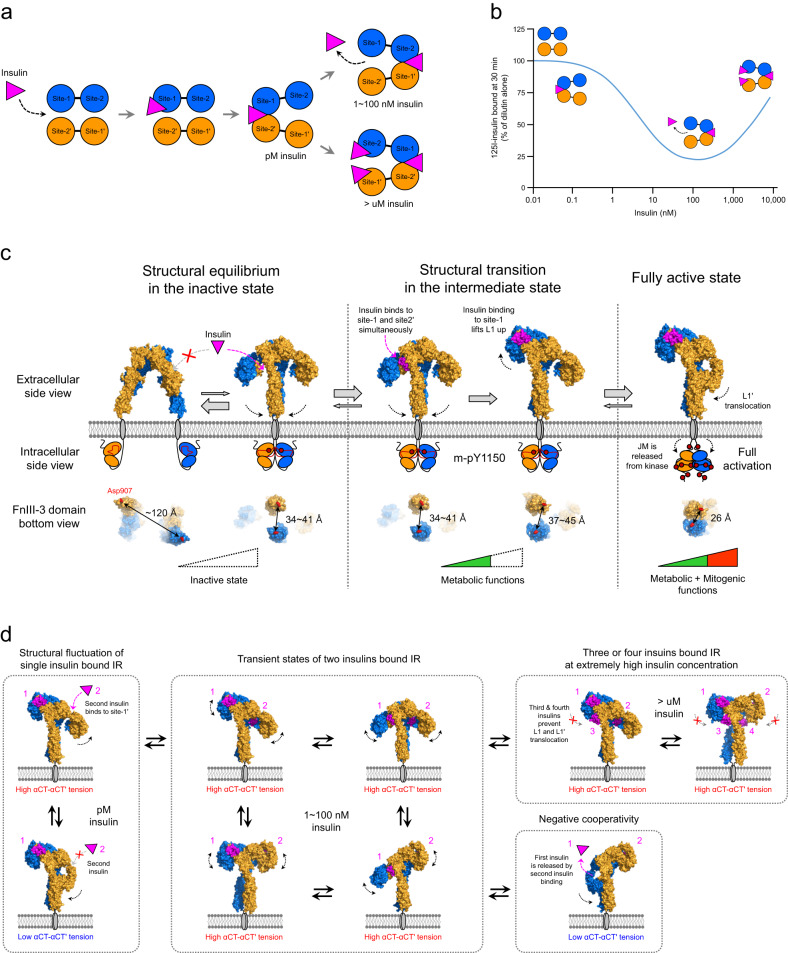
Fig. 6. The proposed model for insulin-induced IR activation and negative cooperativity of insulin binding.
a A conventional bivalent-crosslinking model showing the insulin binding mechanism50,63. At low insulin concentrations (~pM), the first insulin molecule binds strongly to the IR dimer by simultaneously interacting with site-1 and site-2’. As the insulin concentration increases (1–100 nM), the dissociation of previously bound insulin from IR is accelerated by a second insulin molecule binding to the opposite site-1’ and site-2 (negative cooperativity). At very high insulin concentrations (>μM), the binding of the second and third insulin molecule to each site-1’ and site-2 prevents the dissociation of the previously bound insulin from IR. b Dose‒response curve for the negative cooperativity of insulin binding to IR59,60. The bell-shaped curve indicates that accelerated dissociation of bound insulin changes in a concentration-dependent manner. c Stepwise activation model for insulin receptor activation from the extracellular and intracellular views. Insulin is pink, and arrows with dotted lines represent critical conformational changes in each step. The FnIII-3 domain bottom view represents the distance between the membrane proximal ends of FnIII-3 and FnIII-3′ in each step. d Model for the negative cooperativity of insulin binding. The dissociation of previously bound insulin is initiated by binding of a second insulin molecule to site-1’ and site-2. Two-insulin-bound IR undergoes transitions among various conformational states due to its intrinsic instability. When two insulin-bound IR structures transition into the Γ-shaped structure, previously bound insulin is dissociated from IR.