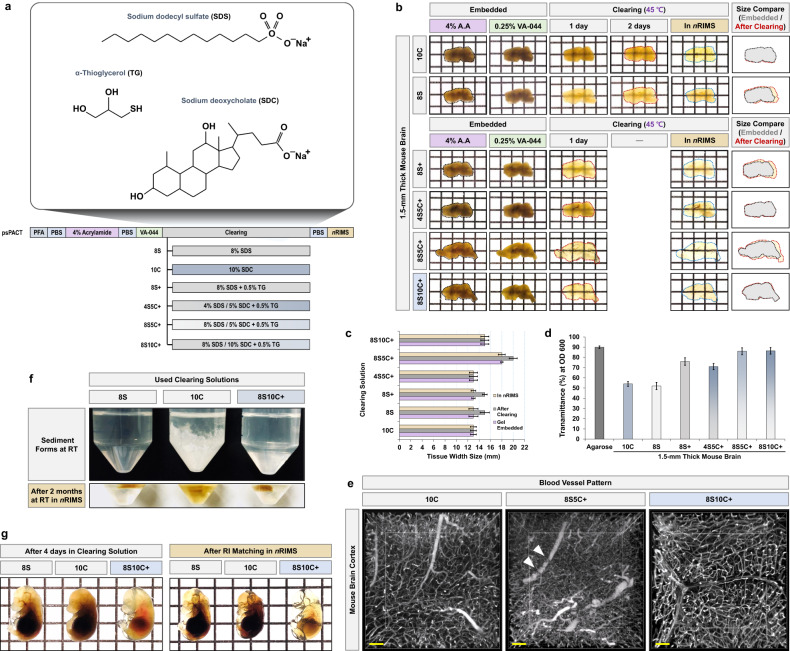
Fig. 1. Generation of transparent mouse brain and embryos using modified PACT.
a Schematic representation of psPACT clearing methods. Clearing solutions were produced by combining three reagents: sodium dodecyl sulfate (SDS), sodium deoxycholate (SDC), and α-thioglycerol (TG). The individual reagents or processes used for polymerization in the passive clearing methods are shown, including the various clearing solutions: 8S (8% SDS), 10S (10% SDS), 8S+ (8% SDS + 0.5% TG), 4S5C+ (4% SDS + 5% SDC + 0.5% TG), 8S5C+ (8% SDS + 5% SDC + 0.5% TG), and 8S10C+ (8% SDS + 10% SDC + 0.5% TG). b Comparison of optical transparency in 1.5-mm-thick mouse brain slices using the psPACT protocol and six different clearing solutions. Right images indicate the sample sizes before (embedded in 0.25% VA-044) and after clearing. Black dotted line (0.25% VA-044), red dotted line (after clearing), and blue dotted line [after refractive index (RI) matching with nRIMS]. c Comparison of brain tissue width in 1% agarose gel (1.5 mm thick) and (b) during the six clearing processes (each n = 3). d Comparison of transmittance (%) of cleared brain samples at OD 600 nm. The results are the averages of three separate tests. e Comparison of lectin immunohistochemical images of mouse brain cortex processed using psPACT with 10C, 8S5C + , and 8S10C+ clearing solutions. The lectin image for each sample was created from serial z-images (25 slices; depth: 500 μm) of the blood vessel pattern obtained by confocal microscopy at 10× magnification (0.45 NA, 2.0 mm working distance), with the microscope focused on a 1 × 1 panel (horizontal × vertical). Scale bar (yellow: 100 μm). f Comparison of the three tissue clearing reagents (8S, 10C, and 8S10C+) and nRIMS solution after incubation of each sample at room temperature. g Comparison of the three clearing solutions in E17.5 mouse embryos after 4 days in 8S (left), 10C (middle), and 8S10C+ (right) clearing solutions. The transparency of all the cleared samples was tested against a patterned background (length × width = 5 × 5 mm).