Abstract
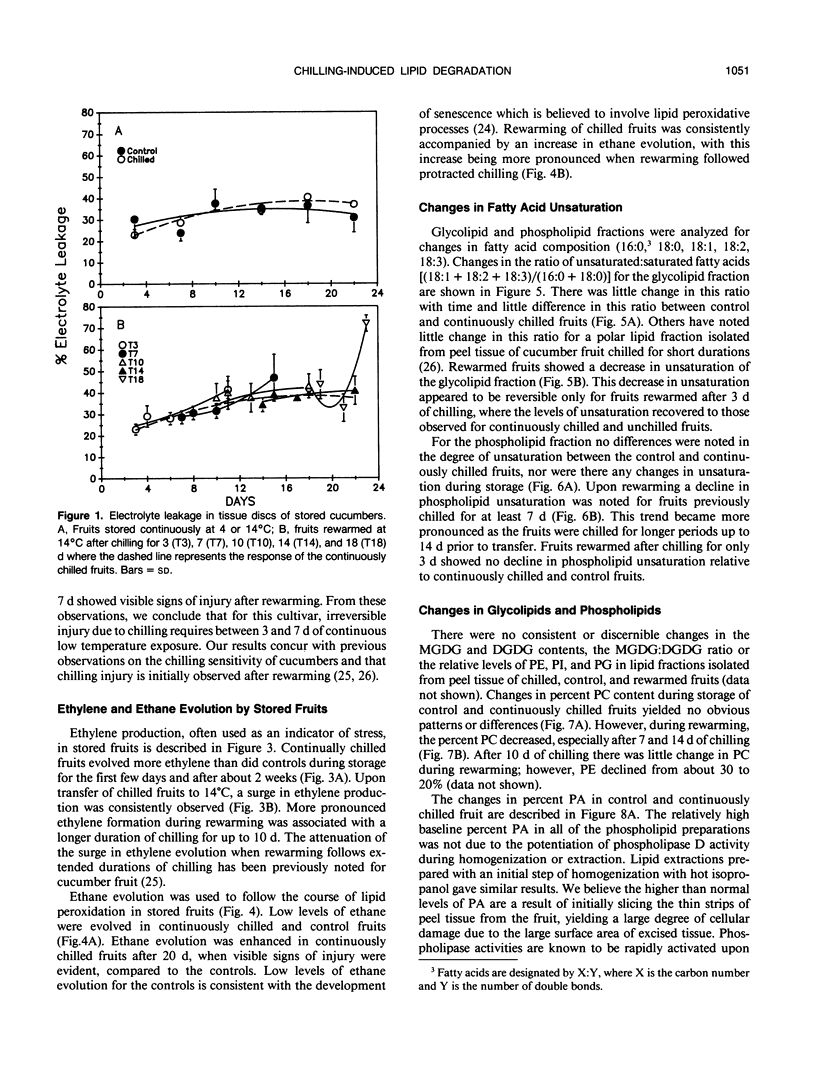
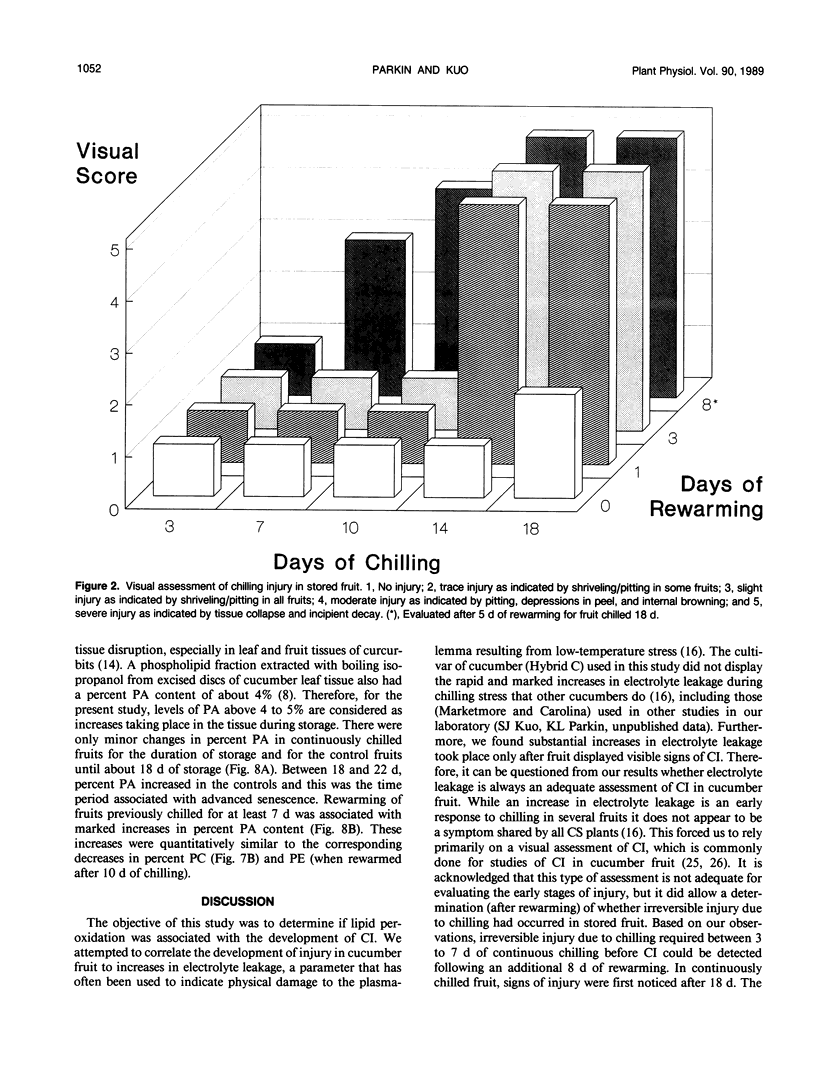
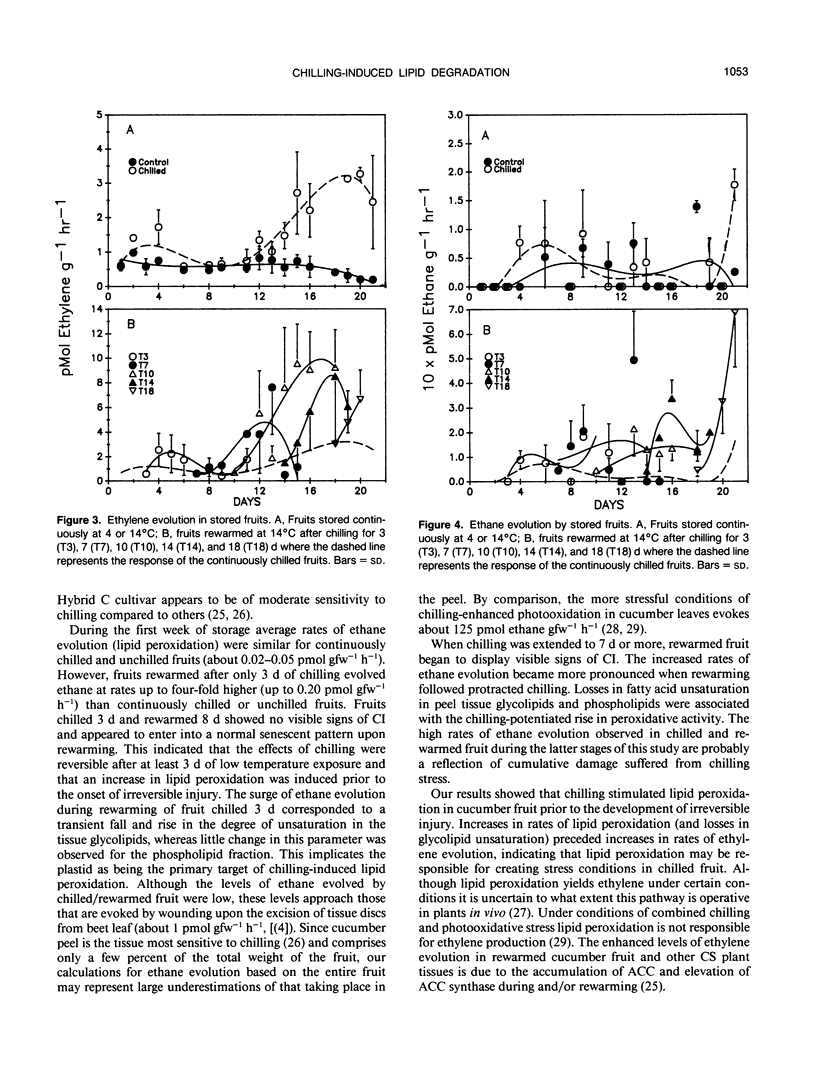
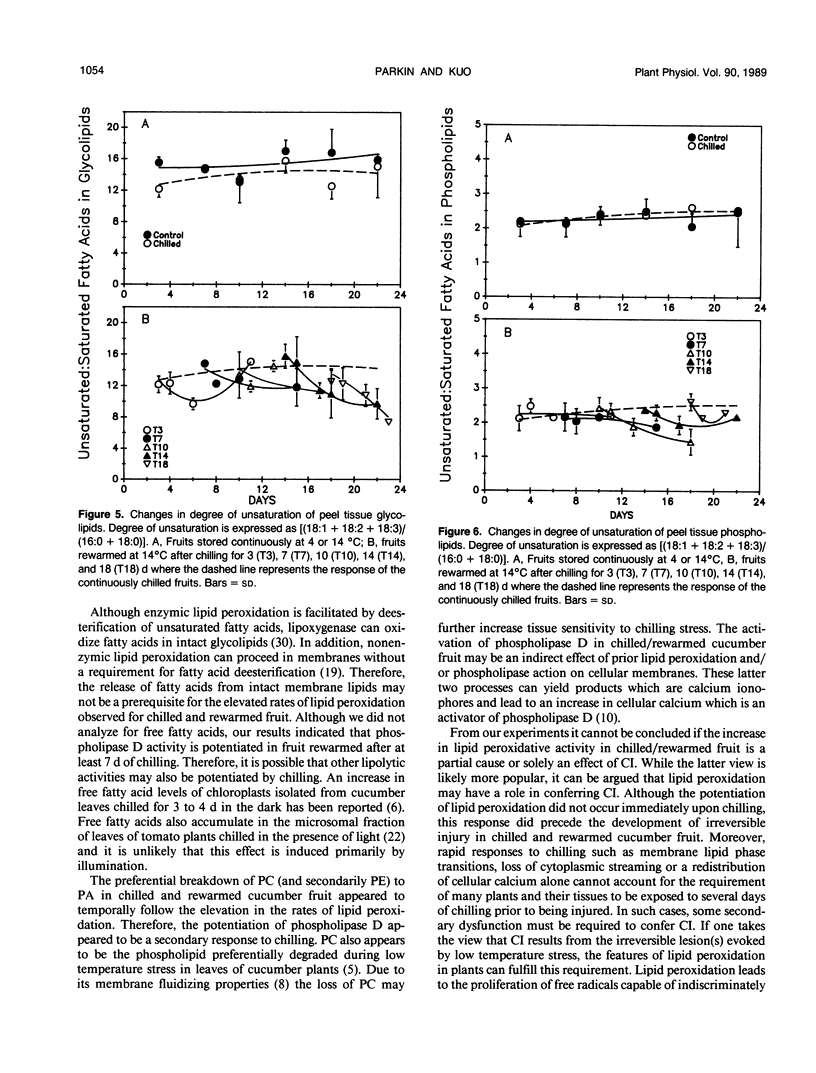
Chilling at 4°C in the dark induced lipid degradation in cucumber (Cucumis sativa L.) fruit upon rewarming at 14°C. Rates of ethane evolution by fruits rewarmed after 3 days of chilling were up to four-fold higher than those evolved by unchilled (14°C) fruits (0.02-0.05 picomoles gram fresh weight−1 hour−1). This potentiation of lipid peroxidation occurred prior to irreversible injury (requiring 3 to 7 days of chilling) as indicated by increases in ethylene evolution and visual observations. Decreases in unsaturation of peel tissue glycolipids were observed in fruits rewarmed after 3 days of chilling, indicating the plastids to be the site of the early phases of chilling-induced peroxidation. Losses in unsaturation of tissue phospholipids were first observed only after chilling for 7 days. Phospholipase D activity appeared to be potentiated in fruits rewarmed after 7 days of chilling as indicated by a decrease in phosphatidylcholine (and secondarily phosphatidylethanolamine) with a corresponding increase in phosphatidic acid. These results indicate that lipid peroxidation may have a role in conferring chilling injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsukov L. I., Victorov A. V., Vasilenko I. A., Evstigneeva R. P., Bergelson L. D. Investigation of the inside-outside distribution, intermembrane exchange and transbilayer movement of phospholipids in sonicated vesicles by shift reagent NMR. Biochim Biophys Acta. 1980 May 8;598(1):153–168. doi: 10.1016/0005-2736(80)90273-4. [DOI] [PubMed] [Google Scholar]
- Gemel J., Kaniuga Z. Comparison of galactolipase activity and free fatty acid levels in chloroplasts of chill-sensitive and chill-resistant plants. Eur J Biochem. 1987 Jul 1;166(1):229–233. doi: 10.1111/j.1432-1033.1987.tb13506.x. [DOI] [PubMed] [Google Scholar]
- Leray C., Pelletier X., Hemmendinger S., Cazenave J. P. Thin-layer chromatography of human platelet phospholipids with fatty acid analysis. J Chromatogr. 1987 Sep 25;420(2):411–416. doi: 10.1016/0378-4347(87)80198-6. [DOI] [PubMed] [Google Scholar]
- Lynch D. V., Steponkus P. L. Plasma Membrane Lipid Alterations Associated with Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1987 Apr;83(4):761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran R. G. Peroxide Levels and the Activities of Catalase, Peroxidase, and Indoleacetic Acid Oxidase during and after Chilling Cucumber Seedlings. Plant Physiol. 1980 Feb;65(2):407–408. doi: 10.1104/pp.65.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter N. A. Chemistry of lipid peroxidation. Methods Enzymol. 1984;105:273–282. doi: 10.1016/s0076-6879(84)05035-7. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Sevanian A., Wratten M. L., McLeod L. L., Kim E. Lipid peroxidation and phospholipase A2 activity in liposomes composed of unsaturated phospholipids: a structural basis for enzyme activation. Biochim Biophys Acta. 1988 Aug 12;961(3):316–327. doi: 10.1016/0005-2760(88)90079-3. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Adams D. O. Chilling-Induced Ethylene Production in Cucumbers (Cucumis sativus L.). Plant Physiol. 1982 Feb;69(2):424–427. doi: 10.1104/pp.69.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. R., Naylor A. W. Chilling-enhanced photooxidation : evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987 Feb;83(2):278–282. doi: 10.1104/pp.83.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]


