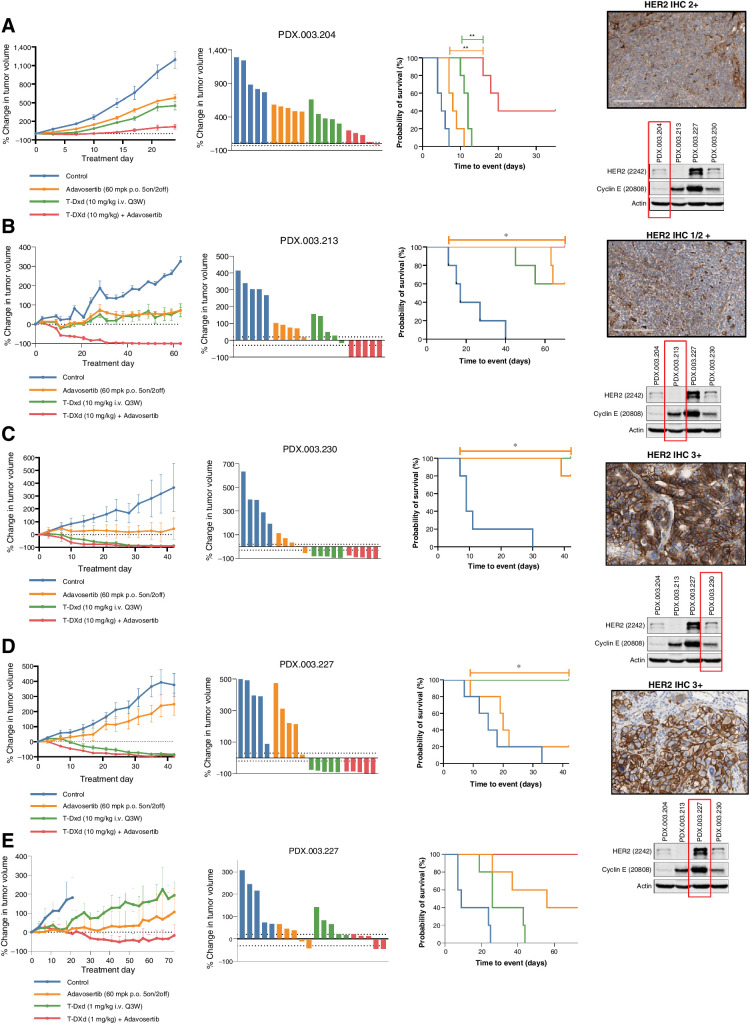
Figure 2.
In vivo activity of T-DXd in combination with adavosertib in HER2 low (1/2+) and high (3+) expressing gastroesophageal cancers. A, Adavosertib (60 mg/kg p.o. 5on/2off) enhanced tumor growth inhibition when combined with T-DXd (10 mg/kg i.v. every 3 weeks) in a HER2 low, cyclin E amplified gastroesophageal cancer PDX model PDX.003.204. Right panel demonstrates HER2 IHC and immunoblotting for HER2, Cyclin E1, and actin. B, Adavosertib (60 mg/kg p.o. 5on/2off) in combination with T-DXd (10 mg/kg i.v. every 3 weeks) led to enhanced tumor regression in a HER2 low, cyclin E amplified gastroesophageal cancer PDX model PDX.003.213, with an ERBB2 V777 L and G778A mutation. C, T-DXd (10 mg/kg IV every 3 weeks) induced durable tumor regression alone and in combination with adavosertib in an ERBB2 amplified and HER2 overexpressing gastroesophageal PDX cancer model PDX.003.230. Adavosertib alone also demonstrated significant antitumor activity. D and E, T-DXd (10 mg/kg i.v. every 3 weeks) induced durable tumor regression in an ERBB2 amplified and HER2 overexpressing gastroesophageal PDX cancer model PDX.003.227 with concomitant cyclin E amplification/expression alone and when T-DXd was combined with adavosertib (60 mg/kg p.o. 5on/2off) (D). Antitumor activity of lower dose T-DXd (1 mg/kg i.v. every 3 weeks) was enhanced with the combination with adavosertib (60 mg/kg p.o. 5on/2off) (E). PDX, patient-derived xenograft; T-DXd, trastuzumab deruxtecan.