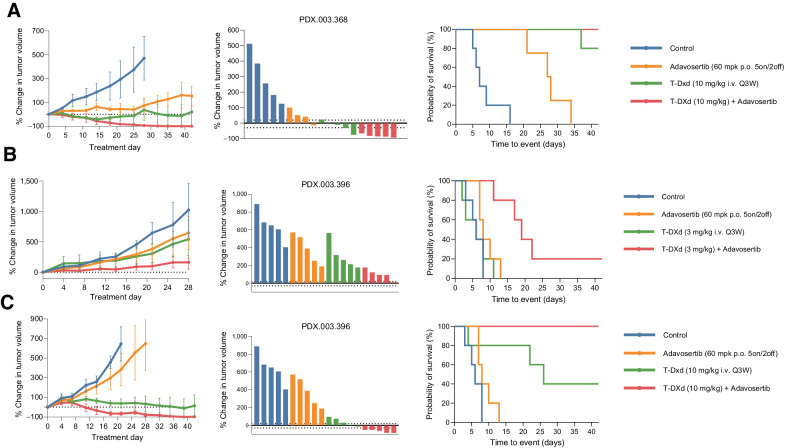
Figure 3.
In vivo activity of T-DXd in combination with adavosertib in other HER2 high (3+) expressing tumors. A, T-DXd (3 mg/kg i.v. every 3 weeks) induced durable tumor regression in an ERBB2 amplified/HER2 overexpressing and CCNE1 amplified endometrial neuroendocrine carcinoma PDX, PDX.003.368. B and C, Adavosertib (60 mg/kg p.o. 5on/2off) in combination with T-DXd (3 and 10 mg/kg i.v. every 3 weeks) led to enhanced tumor regression in a ERBB2 amplified/HER2 overexpressing colorectal cancer model PDX.003.396, which was developed from a patient who had progressed after treatment with T-DXd. PDX, patient-derived xenograft; T-DXd, trastuzumab deruxtecan.