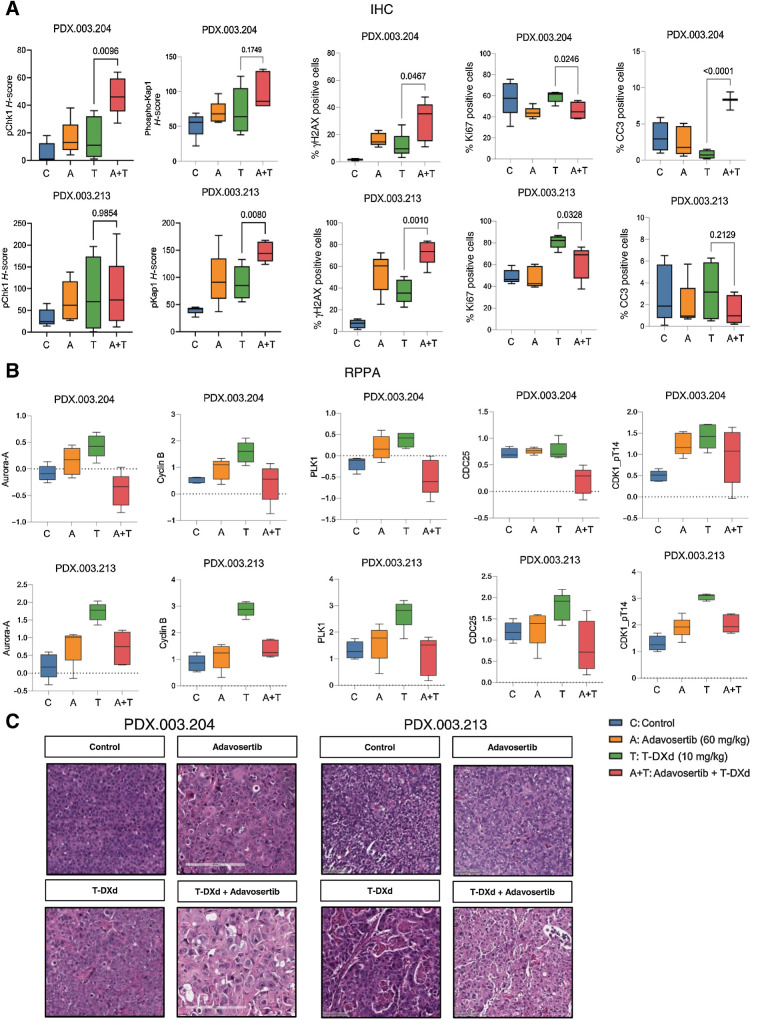
Figure 4.
Pharmacodynamic effects of adavosertib in combination with T-DXd following 10-day treatment. Mice were treated for 10 days with adavosertib, T-DXd, or T-DXd + adavosertib, or were untreated (N = 5). A, PD marker assessment by IHC for adavosertib, T-DXd, and adavosertib + T-DXd tumors assessing phosphorylated Chk1, phosphorylated Kap1, γH2AX, Ki67, and cleaved caspase 3 (CC3). B, Differential expression of proteins assessed by RPPA revealed alterations in expression of several proteins involved in G2–M transition, including Aurora-A, cyclin B, PLK1, CDC25, and phosphorylated CDK1. C, Morphologic features on H&E staining observed following 10-day treatments with adavosertib, T-DXd, or T-DXd + adavosertib. CCNE1, Cyclin E; ERBB2, Erb-B2 receptor tyrosine kinase 2; H&E, hematoxylin and eosin; PD, pharmacodynamic.