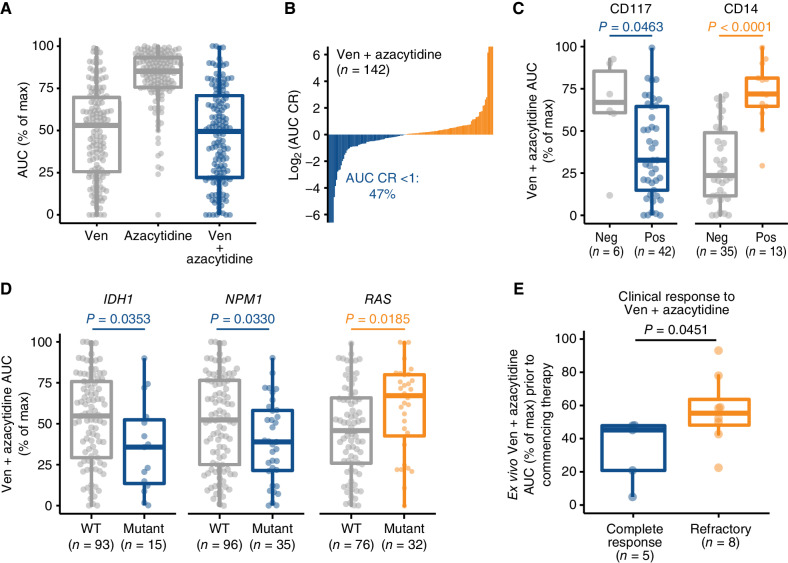
Figure 1.
Ex vivo drug-sensitivity recapitulates clinical experience with Ven + azacytidine in AML. A,Ex vivo sensitivities for venetoclax (Ven), azacytidine and Ven + azacytidine on matched AML primary samples (n = 142). Sensitivity is represented as % of the maximum (max) area-under-the-dose response curve (AUC) derived for a 7-point concentration series ranging from 10 μmol/L to 10 nmol/L. B, Distribution of combination ratio (CR) values for patient samples treated with Ven + azacytidine ex vivo. CR is defined as the AUC of the combination divided by the AUC of the most potent single agent, where AUC CR <1 denotes the enhanced efficacy of the combination. C, Differences in Ven + azacytidine ex vivo sensitivity among patient samples with respect to expression of select immunophenotypic markers of primitive (CD117) and monocytic tumor cells (CD14). Neg, negative; Pos, positive. D, Differences in ex vivo sensitivity of Ven + azacytidine among patient samples based on the presence of prognostically relevant mutations in IDH1, NPM1, and either NRAS or KRAS. WT, wild-type. E, For 10 newly diagnosed patients with AML with both ex vivo screening and subsequent clinical treatment with Ven + azacytidine, ex vivo sensitivity collected at baseline prior to treatment is compared based on subsequent clinical response achieved on treatment. P values shown were determined using Mann–Whitney tests.