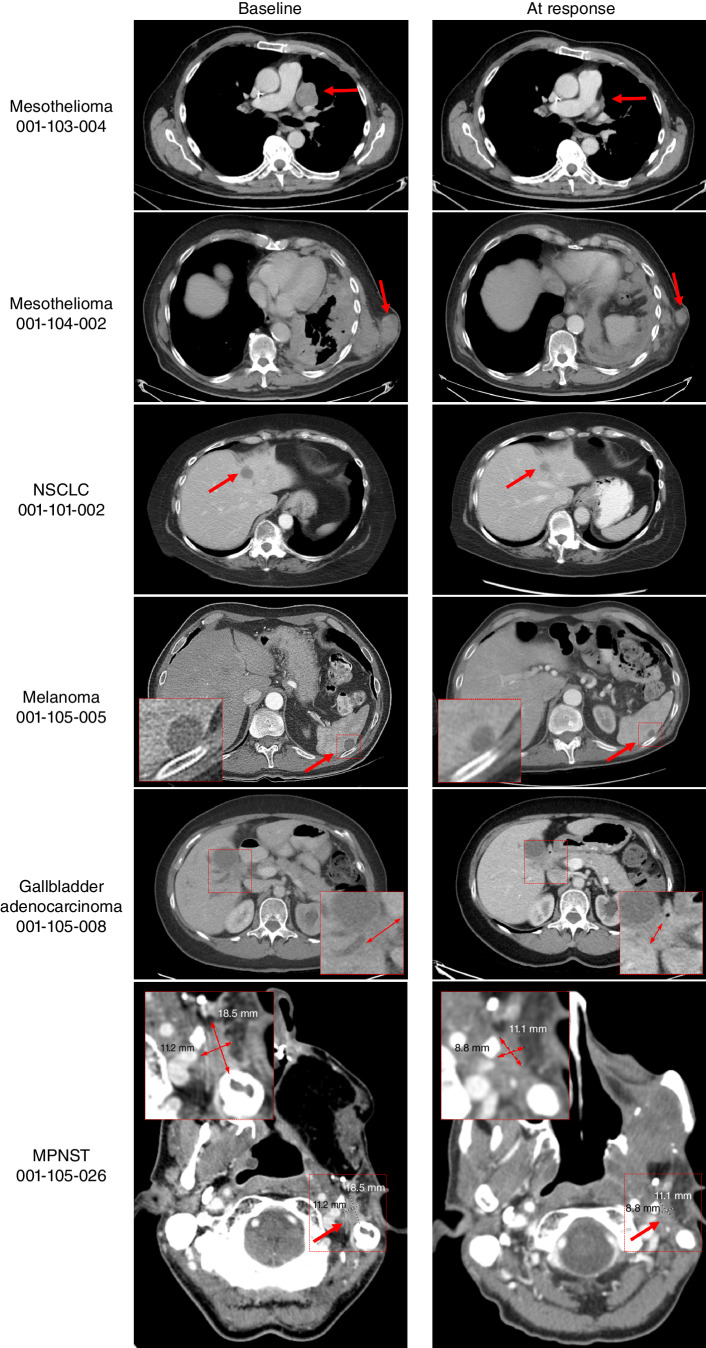
Figure 6.
Activity of MRTX1719 in patients with MTAP del cancers. Mesothelioma patient 001-103-004: Pretreatment and follow-up (FU) C6D20 scans, indicating 56% reduction of target lesions, including the pleural disease shown here. The patient continues on study. Mesothelioma patient 001-104-002: Activity of MRTX1719 in patient epithelioid mesothelioma with MTAP deletion. Pretreatment and FU 3 scans, indicating 30% reduction of target lesions, including soft tissue lesions shown here. The patient continues on study. NSCLC patient 001-101-002: Baseline: dominant mass left hepatic dome measuring 29 × 19 mm, previous 20 × 19 mm. Stable other tiny scattered hypodense hepatic lesions too small to accurately characterize. C5D1: left hepatic dome metastasis, measuring 17 × 17 mm. Stable and mild decreasing size of hepatic metastases. The patient continues on study. Melanoma patient 001-105-005: Baseline and cycle 9 scans of a patient with MTAP del melanoma. Partial response was confirmed at cycle 9, and the patient continues on study. Gallbladder adenocarcinoma patient 001-105-008: Pretreatment and cycle 9 scans of a patient with treatment-refractory MTAP loss gallbladder indicating 43% reduction of target lesions. The patient continues on study. MPNST patient 001-105-026: Patient received MRTX1719 800 mg q.d. Restaging after 4 cycles shows a partial response with a 38.9% decrease of target lesions. The patient continues on study.