Abstract
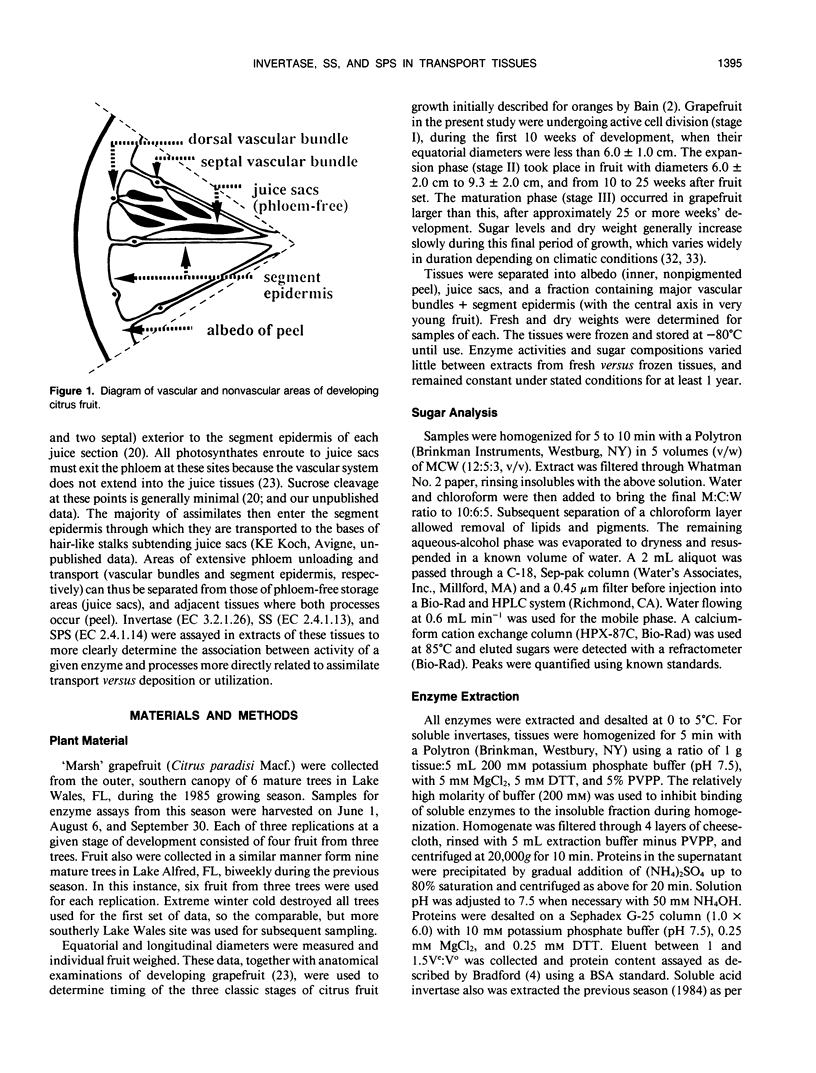
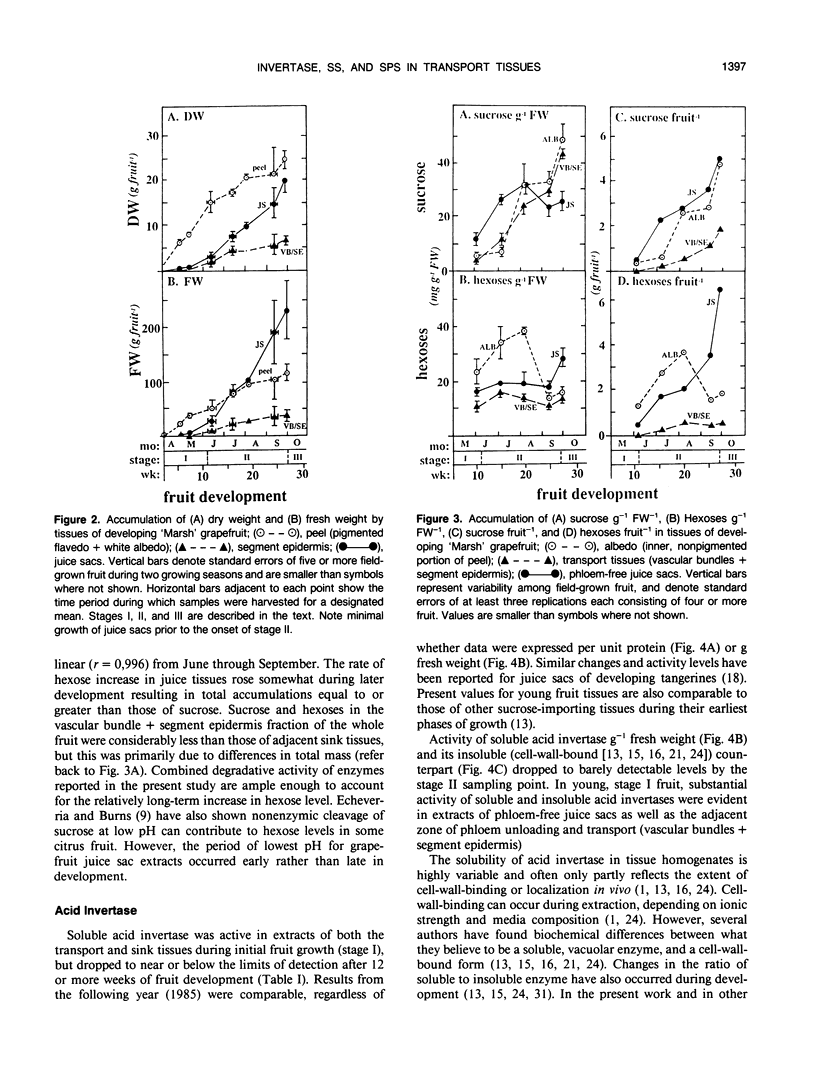
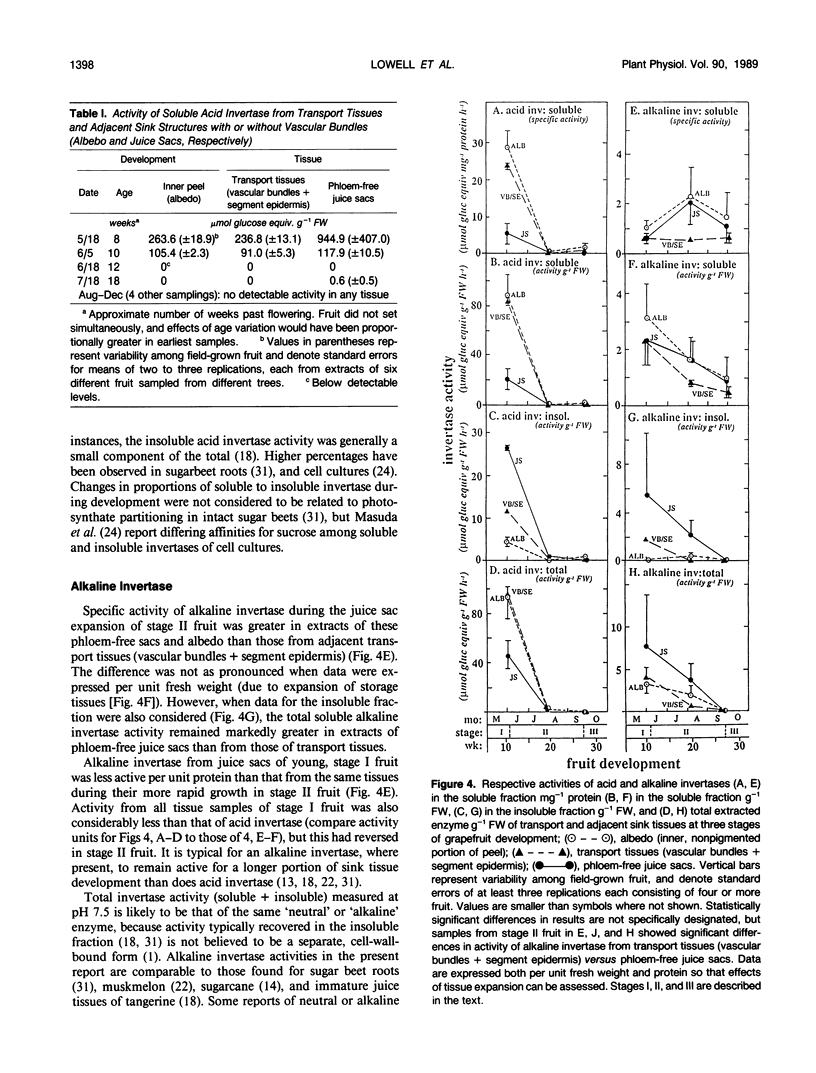
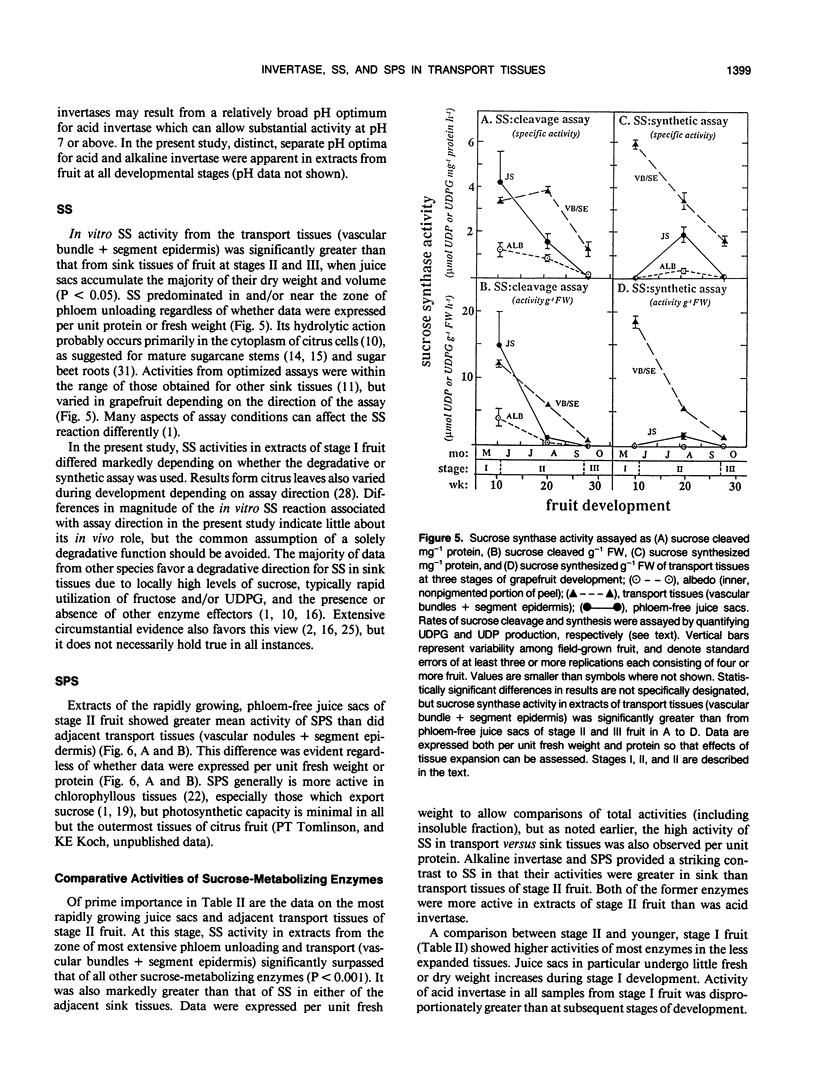
Juice tissues of citrus lack phloem; therefore, photosynthates enroute to juice sacs exit the vascular system on the surface of each segment. Areas of extensive phloem unloading and transport (vascular bundles + segment epidermis) can thus be separated from those of assimilate storage (juice sacs) and adjacent tissues where both processes occur (peel). Sugar composition, dry weight accumulation, and activities of four sucrose-metabolizing enzymes (soluble and cell-wall-bound acid invertase, alkaline invertase, sucrose synthase, and sucrose phosphate synthase) were measured in these transport and sink tissues of grapefruit (Citrus paradisi Macf.) to determine more clearly whether a given enzyme appeared to be more directly associated with assimilate transport versus deposition or utilization. Results were compared at three developmental stages. Activity of sucrose (per gram fresh weight and per milligram protein) extracted from zones of extensive phloem unloading and transport was significantly greater than from adjacent sink tissues during the stages (II and III) when juice sacs grow most rapidly. In stage II fruit, activity of sucrose synthase also significantly surpassed that of all other sucrose-metabolizing enzymes in extracts from the transport tissues (vascular bundles + segment epidermis). In contrast, sucrose phosphate synthase and alkaline invertase at this stage of growth were the most active enzymes from adjacent, rapidly growing, phloem-free sink tissues (juice sacs). Activity of these two enzymes in extracts from juice sacs was significantly greater than that form the transport tissues (vascular bundles + segment epidermis). Soluble acid invertase was the most active enzyme in extracts from all tissues of very young fruit (stage I), including nonvascular regions, but nearly disappeared prior to the onset of juice sac sugar accumulation. The physiological function of high sucrose synthase activity in the transport tissues during rapid sucrose import remains to be determined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Echeverria E., Burns J. K. Vacuolar Acid hydrolysis as a physiological mechanism for sucrose breakdown. Plant Physiol. 1989 Jun;90(2):530–533. doi: 10.1104/pp.90.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Sacher J. A., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. I. Studies on Enzymes of the Cycle. Plant Physiol. 1963 May;38(3):338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986 Aug;81(4):1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Blanchette J. T., Okita T. W. Wheat invertases : characterization of cell wall-bound and soluble forms. Plant Physiol. 1985 Jun;78(2):241–245. doi: 10.1104/pp.78.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle S. E., Dunlap J. R. Sucrose Metabolism in Netted Muskmelon Fruit during Development. Plant Physiol. 1987 Jun;84(2):386–389. doi: 10.1104/pp.84.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Takahashi T., Sugawara S. Acid and alkaline invertases in suspension cultures of sugar beet cells. Plant Physiol. 1988 Jan;86(1):312–317. doi: 10.1104/pp.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M., Copeland L. Sucrose synthase of soybean nodules. Plant Physiol. 1985 May;78(1):149–154. doi: 10.1104/pp.78.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalstig J. G., Hitz W. D. Transport and Metabolism of a Sucrose Analog (1'-Fluorosucrose) into Zea mays L. Endosperm without Invertase Hydrolysis. Plant Physiol. 1987 Dec;85(4):902–905. doi: 10.1104/pp.85.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. C. Movement of C-Labeled Assimilates into Kernels of Zea mays L: II. Invertase Activity of the Pedicel and Placento-Chalazal Tissues. Plant Physiol. 1972 Feb;49(2):203–206. doi: 10.1104/pp.49.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. E., Snyder F. W. Comparative Enzymic Studies of Sucrose Metabolism in the Taproots and Fibrous Roots of Beta vulgaris L. Plant Physiol. 1979 Dec;64(6):1070–1073. doi: 10.1104/pp.64.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M., Maretzki A. Group translocation as a mechanism for sucrose transfer into vacuoles from sugarcane cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4697–4701. doi: 10.1073/pnas.82.14.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]


