Fig. 3. H1ssF elicits broad antibody responses to group 1 influenza HA antigens.
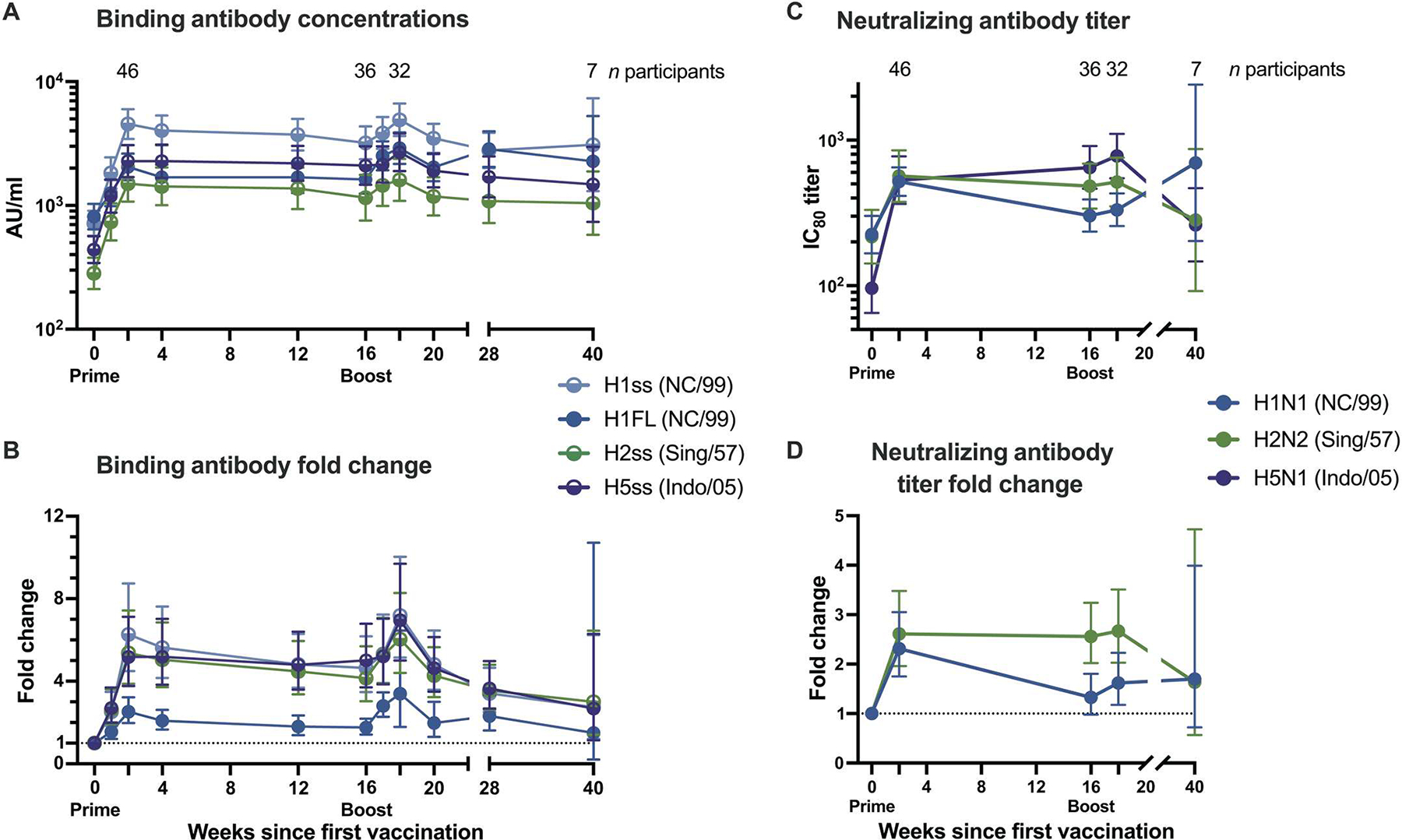
(A) Geometric means and 95% confidence intervals (CIs) are shown for all 60-μg dose recipients’ binding antibody concentrations. (B) Fold change of binding antibody concentrations from baseline for group 1 influenza virus antigens. Binding was assessed by ECLIA. (C) Neutralizing IC80 antibody titers for three viruses and (D) fold change from baseline for two viruses, as assessed by reporter-based microneutralization assay. Half circles in (A) and (B) denote stabilized stem (ss) antigens. Dotted lines in (B) and (D) indicate baseline. In (D), H5N1 fold change calculations could not be performed because of low baseline titers. Exact numbers of participant samples analyzed at each time point are listed in table S4. AU, arbitrary units; NC/99, A/New Caledonia/20/1999; Sing/57, A/Singapore/1/1957; Indo/05, A/Indonesia/5/2005.
