Abstract
Objective
Catheter-associated urinary tract infections (CAUTI) are common worldwide, but due to limited resources, its actual burden in low-income countries is unknown. Currently, there are gaps in knowledge about CAUTI due to lack of surveillance activities in Sierra Leone. In this prospective cohort study, we aimed to determine the incidence of CAUTI and associated antibiotic resistance in two tertiary hospitals in different regions of Sierra Leone.
Results
The mean age of the 459 recruited patients was 48.8 years. The majority were females (236, 51.3%). Amongst the 196 (42.6%) catheterized patients, 29 (14.8%) developed CAUTI. Bacterial growth was reported in 32 (84%) patients. Escherichia coli (14, 23.7%), Klebsiella pneumoniae (10, 17.0%), and Klebsiella oxytoca (8, 13.6%) were the most common isolates. Most isolates were ESBL-producing Enterobacteriaceae (33, 56%) and WHO Priority 1 (Critical) pathogens (38, 71%). Resistance of K. pneumoniae, K. oxytoca, E. coli, and Proteus mirabilis was higher with the third-generation cephalosporins and penicillins but lower with carbapenems, piperacillin-tazobactam and amikacin. To reduce the high incidence of CAUTI and multi-drug resistance organisms, urgent action is needed to strengthen the microbiology diagnostic services and develop and implement catheter bundles that provide clear guidance for catheter insertion, care and removal.
Keywords: Multidrug resistance organisms (MRO), Extended spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, Catheter-associated urinary tract Infections (CAUTI), WHO priority pathogens, Carbapenem resistance Enterobacteriaceae (CRE)
Introduction
Catheter-associated urinary tract infections (CAUTI) are common worldwide, but due to limited resources, their actual burden in low-income countries is unknown [1]. However, global estimates report that low-income countries have a higher CAUTI burden than high-income countries, with cumulative incidence densities of 8.8/1000 catheter days and 4.1/1000 catheter days, respectively [1]. In a study conducted on healthcare-associated infections (HAIs) in Ethiopia, CAUTI was the most commonly detected HAI [2].
Urinary catheters are critical in routine health care delivery, but can lead to CAUTI if not handled properly. There are several adverse events associated with CAUTI, including prolonged hospital stay, worsening in-hospital mortality, and increased healthcare costs [3–7]. Furthermore, many of the bacteria causes of CAUTI are multidrug-resistant pathogens, including extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae [8, 9]. Despite these challenges, systematic support for the routine diagnosis of CAUTI and related antibiotic resistance is limited by the lack of adequate surveillance activities in some African countries [10].
It is in this context that the World Health Organization (WHO) developed a global initiative to support low- and middle-income countries in strengthening their surveillance structures for CAUTI and other HAIs [11]. However, the financial and human resources challenges hinder efforts to provide a safe hospital environment for populations in low-income countries [12].
Sierra Leone, a low-income country in West Africa, has been affected by major public health emergencies, including the largest Ebola outbreak to date [13]. Before the 2014–2016 Ebola outbreak, the country had no system to prevent HAIs [14]. Consequently, the government developed a policy to guide the implementation of infection prevention and control (IPC) practices, including the surveillance and prevention of HAIs [14]. However, this development has not brought significant changes to the surveillance of HAIs in the country. Although the Ministry of Health and its partners have begun surveillance of surgical site infections in some hospitals, Sierra Leone does not have a CAUTI surveillance system. Currently, there are gaps in knowledge about CAUTI due to limited surveillance activities in the country. At the time of writing, no studies have been published on the incidence of CAUTI in Sierra Leone. This situation, and the fact that our previous work has shown deep-rooted challenges in the implementation of IPC practices in Sierra Leone prompted us to pilot a surveillance program for CAUTI in two hospitals situated in two geographic regions of Sierra Leone [15–17]. In this study, we aimed to determine the incidence of CAUTI and associated antibiotic resistance in order to inform preventive interventions.
Methods
Study design
The study utilized a prospective cohort design involving primary data collection.
Study settings
Sierra Leone is divided into five geographic regions, including the Northern province and the Western Area. The Western Area is the most densely populated region of Sierra Leone with a population of about 1.5 million [18].
The public health system is divided into primary, secondary and tertiary levels of care. The national and regional hospitals provide tertiary care. There are 25 public hospitals in Sierra Leone of which 9 provide tertiary services. The study was conducted in two of the tertiary/regional hospitals (Makeni Government Hospital: MGH and the 34 Military Hospital: MH) in different geographic regions of Sierra Leone as both are likely representative of many tertiary hospitals in sub-Saharan Africa. Both hospitals serve large populations in their catchment areas. While MH is located in Freetown, MGH is about 170 km from the capital with a catchment population of 606,544 (approximately 8.6% of the Sierra Leonean population). Both hospitals have similar infrastructure with bed capacities of 207 (regional) and 187 (capital city). They both provide medical services [18, 19].
Study population and sampling technique
All consenting patients aged 18 years or older admitted on the medical wards of the two hospitals were enrolled in the study between March and October 2021. A sample size of 459 patients was reached over a 24-week sampling period.
A team, comprised of trained nurses on the detection of HAIs collected the required information. Patients were recruited in medical wards or intensive care units (ICU) at admission and followed until the end of admission. Sociodemographic variables, clinical parameters and information on urinary catheterization were recorded at baseline. After the baseline assessment, the patients were then monitored for features suggestive of CAUTI.
In cases of suspected CAUTI, we aseptically collect urine for urinalysis, culture, and sensitivity to diagnose CAUTI. We define CAUTI as ‘the presence of at least one of the following signs or symptoms: fever (> 38.0°C), suprapubic pain or tenderness, costovertebral angle pain or tenderness, dysuria, and urinary frequency in a patient that had an indwelling urinary catheter that had been in place for more than 2 consecutive days in an inpatient location and the presence of a positive dipstick for leukocyte esterase and/or nitrite and microorganisms seen on Gram’s stain of unspun urine and a positive urine culture of ≥ 105 colony-forming units/ml with no more than two bacteria species’ [20, 21].
Laboratory procedure
Urine samples were streaked onto the chromogenic agar plate and incubated aerobically at 37 °C for 18 to 24 h. A single bacterial colony was picked up where there is growth and then streaked onto a Brain Heart Infusion agar plate for Gram stain. Bacteria solutions were prepared to 0.5–0.63 McFarland turbidity standards in polystyrene tubes (bioMérieux, France) using a DensiCHEK Plus turbidimeter (bioMérieux, France). A suspension of each isolate was loaded onto the VITEK 2 compact system and incubated overnight at 37 °C to detect antibiotic susceptibility. Escherichia coli, Klebsiella spp., Serratia ficario and Proteus mirabilis were tested for susceptibility to cephalosporins alone and in combination with clavulanic acid. A reduction in the proportion of bacterial growths in wells containing both cephalosporin and clavulanic acid compared with wells containing cephalosporin alone was considered indicative of extended-spectrum β-lactamase (ESBL) production. Carbapenemase production was determined by analysis of carbapenem susceptibility results. Isolates were defined as susceptible, intermediate, or resistant based on the minimum inhibitory concentrations of imipenem and meropenem in the Clinical Laboratory Standard Institute (CLSI) breakpoints updated in January 2020 [22]. All culture and resistance test results are sent to research teams and service providers within 24 h.
Data management and analysis
All the data were recorded electronically on a password protected Epicollect software platform accessible only to the study team and exported to excel format. Data was clean, coded and analyzed using the Statistical Package for Social Sciences version 21.0 (Armonk, NY: IBM Corp). Descriptive statistics such as frequencies and percentages were used to present demographic, clinical characteristics of study participants and antibiotic resistance profile.
Results
Demographic characteristics of study participants
Most of the study participants were treated in MH (245, 53.4%). There were 236 (51.3%) female patients. The mean age was 48.8 years (SD, 18.0) (Table 1).
Table 1.
Demographic characteristics of study participants
| Parameter | Total N(%) |
MH n(%) |
MGH n(%) |
|---|---|---|---|
| Overall total | 459(100) | 245(53.4) | 214(46.6) |
| Sex | |||
| Female | 236(51.4) | 122(49.8) | 114(53.3) |
| Male | 223(48.6) | 123(50.2) | 100(46.7) |
| Age(yrs) | |||
| Mean (SD) | 48.8(18.0) | 53.3(15.9) | 43.7(18.8) |
| Marital status | |||
| Single | 102(22.2) | 47(19.2) | 55(25.7) |
| Married | 291(63.4) | 159(64.9) | 132(61.7) |
| Separated/divorced/widowed | 66(14.4) | 39(15.9) | 27(12.6) |
| Education | |||
| None | 192(41.8) | 57(23.3) | 135(63.1) |
| Primary | 29(6.3) | 18(7.3) | 11(5.1) |
| Secondary | 159(34.6) | 111(45.3) | 48(22.4) |
| Tertiary | 79(17.2) | 59(24.1) | 20(9.4) |
| Occupation | |||
| None | 74(16.1) | 37(15.1) | 37(17.3) |
| Student | 44(9.6) | 19(7.8) | 25(11.7) |
| Informal sector | 213(46.4) | 85(34.7) | 128(59.8) |
| Formal sector | 82(17.9) | 61(24.9) | 21(9.8) |
| Retired | 46(10.0) | 43(17.5) | 3(1.4) |
| Social history | |||
| Never smoked, never taken alcohol | 372(81.1) | 203(82.9) | 169(79.0) |
| Only smokes | 23(5.0) | 13(5.3) | 10(4.7) |
| Only takes alcohol | 19(4.1) | 9(3.7) | 10(4.7) |
| Smokes and drinks alcohol | 45(9.8) | 20(8.2) | 25(11.7) |
MH = 34 Military hospital; MGH = Makeni government hospital
Clinical characteristics, catheterization and development of CAUTI
Twenty-seven (8.1%) patients were admitted to ICU. Overall, 96(21%) patients had been admitted in a hospital in the preceding 3 months. Fever occurred in 24 (12.2%) patients.
One hundred and ninety-six (42.7%) patients were either admitted with a urine catheter or had one placed during the course of their admission. Although the reason for catheter placement was not stated in 21 (11%) cases, the commonest (95, 48.5%) reason for urinary catheter placement was urinary incontinence. Urinary catheter was in situ for a mean (SD) number of 4 (2) days, and was in place for over 3 days in 87(44.4%) patients. Of 196 catheterized patients, 38 (19.4%) had suspected CAUTI, with fever being the most common feature (Table 2). On urinalysis, 36(18.6%) patients either had a positive leucocyte esterase and/or nitrite in their urine (Table 2).
Table 2.
Clinical characteristics and findings on the urinalysis of the study participants
| Parameter | Total N(%) |
MH n(%) |
MGH n(%) |
|---|---|---|---|
| Ward | |||
| Medical | 422(91.9) | 244(99.6) | 178(83.2) |
| ICU | 37(8.1) | 1(0.4) | 36(16.8) |
| Previous hospital admission in last three months | |||
| No | 362(79.0) | 214(87.3) | 148(69.5) |
| Yes | 96(21.0) | 31(12.7) | 65(30.5) |
| Symptoms*Φ | |||
| Fever | 24(12.2) | 10(8.5) | 14(18.0) |
| Suprapubic pain | 21(10.7) | 13(11.0) | 8(10.2) |
| Urinary frequency | 22(11.2) | 11(9.3) | 11(14.1) |
| Dysuria | 15(7.6) | 10(8.4) | 5(6.4) |
| Loin pain | 5(2.6) | 4(3.4) | 1(1.3) |
| Presence of urinary catheter | |||
| No | 263(57.3) | 127(51.8) | 136(63.5) |
| Yes | 196(42.7) | 118(48.2) | 78(36.5) |
| Reasons for catheterization * | |||
| Urinary retention | 41(20.9) | 23(19.5) | 18(23.1) |
| Urinary incontinence | 95(48.5) | 87(73.7) | 8(10.3) |
| Ambulatory dysfunction | 39(19.9) | 0 | 39(50) |
| Reason not stated | 21(10.7) | 8(6.8) | 13(16.7) |
| Suspected CAUTI * | 38(19.4) | 24(20.3) | 14(17.9) |
| CAUTI * | 29(14.8) | 19 (16.1) | 10(12.8) |
| Findings on urinalysis | |||
| Leucococyte estrase | 26(13.3) | 15 (75.0) | 11 (84.6) |
| Nitrite | 3 (1.5) | 1(5.0) | 2(15.4) |
| Leucocyte estrase plus nitrite | 7(3.6) | 4(20.0) | 0(0.0) |
*Denominator is number in whom urinary catheter was present (i.e. 196). ΦMultiple answers
MH = 34 Military Hospital MGH = Makeni Government Hospital CAUTI = catheter-associated urinary tract infection
Bacterial isolates in patients with CAUTI
A significant bacterial growth of ≥105 colony-forming units/ml was reported in 32 patients with suspected CAUTI, giving a bacterial growth rate of 84%; 71% (10/14) for MGH and 92% (22/24) for MH. Among the 32 patients with a significant bacteriuria, 3 had more than two species of bacteria. Therefore, the CAUTI incidence is 14.8%.
A total of 59 bacterial isolates were reported for MH (38, 64.4%) and MGH (21, 35.6%). Escherichia coli (14, 23.7%), Klebsiella pneumoniae (10, 17.0%), and Klebsiella oxytoca (8, 13.6%) were the most common urinary bacteria isolates from both hospitals. Enterococcus spp. (4, 10.5%), Coagulase Negative Staphylococcus spp. (4, 10.5%) and Acinetobacter baumannii complex (2, 3.4%) were only isolated from MH. On the other hand, a single isolate of Proteus mirabilis, Bordetella henzii and Methylobacterium spp. were reported only in MGH (Table 3).
Table 3.
Distribution and rank order of bacterial isolates from the urine of patients with CAUTI
| Bacterial isolate | Rank order | Total no. | MH | MGH | |||
|---|---|---|---|---|---|---|---|
| N = 59 | % | N = 38 | % | N = 21 | % | ||
| Escherichia coli | 1 | 14 | 23.7 | 11 | 29.0 | 3 | 14.3 |
| Klebsiella pneumoniae | 2 | 10 | 17.0 | 7 | 18.4 | 3 | 14.3 |
| Klebsiella oxytoca | 3 | 8 | 13.6 | 6 | 15.8 | 2 | 9.5 |
| Pseudomonas aeruginosa | 4 | 5 | 8.5 | 4 | 10.5 | 1 | 4.8 |
| Rhizobium radiobacter | 4 | 5 | 8.5 | 2 | 5.3 | 3 | 14.3 |
| Coagulase Negative Staphylococcus spp. (haemolyticus = 3 and sciuri = 1) | 5 | 4 | 6.8 | 4 | 10.5 | 0 | 0.0 |
| Enterococcus spp. (faecalis = 2, faecium = 1, casseliflavus = 1) | 5 | 4 | 6.8 | 4 | 10.5 | 0 | 0.0 |
| Acinetobacter baumannii complex | 6 | 2 | 3.4 | 0 | 0.0 | 0 | 0.0 |
| Bockholderia cepacia complex | 6 | 2 | 3.4 | 0 | 0.0 | 2 | 9.5 |
| Sphingomonas paucimobilis | 7 | 1 | 1.7 | 1 | 2.6 | 0 | 0.0 |
| Serratia ficario | 7 | 1 | 1.7 | 0 | 0.0 | 1 | 4.8 |
| Bordetella henzii | 7 | 1 | 1.7 | 0 | 0.0 | 1 | 4.8 |
| Proteus mirabilis | 7 | 1 | 1.7 | 0 | 0.0 | 1 | 4.8 |
| Methylobacterium spp. | 7 | 1 | 1.7 | 0 | 0.0 | 1 | 4.8 |
Patterns of antibiotic resistance of urine isolates
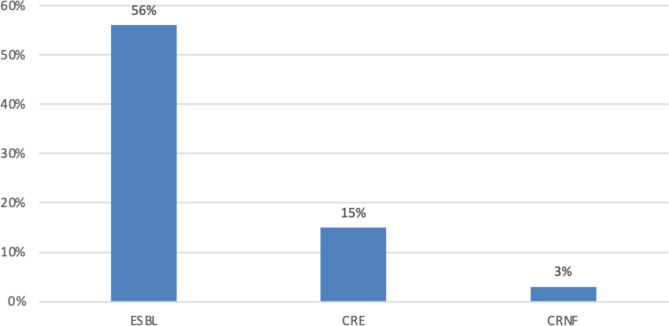
Among the multidrug resistance bacteria, 33 (56%) were ESBL-producing Enterobacteriaceae and 5 (15%) were carbapenem-resistant Enterobacteriaceae (Fig. 1). There were 38 (71%) isolates in the WHO Priority 1 (Critical) Pathogens List, but none of the isolates was in the Priority Pathogens Lists 2 and 3.
Fig. 1.
Patterns of multi-drug resistance of bacteria isolates from patients with CAUTI. ESBL = Extended spectrum β-lactamase producing Enterobacteriaceae CRE = Carbapenem Resistance Enterobacteriaceae CRNF = Carbapenem Resistance Non-lactose fermenters
Resistance to the third-generation cephalosporins, penicillins, aztreonam, tetracyclines, quinolones, and co-trimoxazole was high in K. pneumoniae, K. oxytoca, E. coli, and Proteus mirabilis. In contrast, meropenem, imipenem, piperacillin-tazobactam, amikacin and nitrofurantoin showed relatively low resistance to most isolates (Table 4).
Table 4.
Antibiotic resistance profile of Gram-negative bacteria isolates
| Antibiotics | Resistance rate (%) ® | |||||||
|---|---|---|---|---|---|---|---|---|
|
E. coli
N = 14 |
K. pneumoniae
N = 10 |
S. paucimobilis
N = 1 |
P. aeruginosa
N = 5 |
A.
baumanii N = 2 |
K. oxytoca N = 8 | P. mirabilis N = 1 |
B. cepacia
N = 2 |
|
| Imipenem | 7.10 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | - |
| Meropenem | 7.10 | 20.0 | 0.0 | 0.0 | - | 0.0 | 100.0 | - |
| Ampicillin | 92.9 | - | 100.0 | - | 100.0 | - | - | - |
| Ampicillin-sulbactam | 71.4 | 100.0 | 100.0 | - | 50.0 | 35.0 | - | - |
| Ciprofloxacin | 85.7 | 100.0 | 100.0 | 20.0 | 100.0 | 85.7 | 100.0 | - |
| Amikacin | 7.1 | 100.0 | 100.0 | 0.0 | 100.0 | 7.1 | 0.0 | - |
| Gentamicin | 100.0 | 100.0 | 100.0 | 40.0 | 100.0 | 71.4 | 100.0 | - |
| Tobramycin | 78.6 | 100.0 | 100.0 | 40.0 | 100.0 | 78.6 | 100.0 | - |
| Aztreonam | 100.0 | 100.0 | 100.0 | - | - | 100.0 | - | - |
| Co-trimoxazole | 50.0 | 100.0 | 100.0 | - | 0.0 | 50.0 | - | - |
| Nitrofurantoin | 10.0 | 50.0 | 100.0 | - | 100.0 | 37.5 | - | - |
| Ceftriaxone | 100.0 | 100.0 | 100.0 | - | 100.0 | 100.0 | 100 | - |
| Cefuroxime | 100.0 | 100.0 | 100.0 | - | 100.0 | 100.0 | - | - |
| Cefotetan | 100.0 | 30.0 | 100.0 | - | 100.0 | 21.4 | 100.0 | - |
| Cefazolin | 100.0 | 100.0 | 100.0 | - | 100.0 | 100.0 | - | - |
| Ceftazidime | 85.7 | 90.0 | 100.0 | 20.0 | 100.0 | 50.0 | 100.0 | - |
| Cefepime | 78.6 | 70.0 | 100.0 | 20.0 | 100.0 | 78.6 | 100.0 | - |
| Piperacillin/tazobactam | 28.6 | 30.0 | 100.0 | 00.0 | 0.0 | 35.7 | 100.0 | - |
Discussion
The CAUTI incidence reported in this study is 14.8%, which is almost similar to the CAUTI incidence reported in Ethiopia (16.9%), Uganda (15.3%), and Sudan (16.4%) [23–25]. However, the incidence of CAUTI reported in our study is much higher than reported in high-income countries such as the United States (1.4%), Australia (0.9%), and Italy (6.2%) [26–28]. These findings are expected, considering the fact that many hospitals in sub-Saharan have similar challenges in providing financial and human resources to support IPC and antimicrobial stewardship (AMS) activities and reinforce the fact that regardless of where patients receive care in sub-Saharan, they may have a similar risk of developing CAUTI. To reduce the high incidence of CAUTI, international guidelines and global initiatives have been developed to prevent unnecessary catheterization, but many are not readily available or implemented in low-income countries such as Sierra Leone [29]. This may account for the high rates of CAUTI in our study and other studies in sub-Saharan Africa. Therefore, sub-Saharan African countries need a coordinated approach to prevent CAUTI and ensure the safety of their populations.
In 2017, WHO published a list of priority pathogens for antibiotic resistance, which aims to align antibiotic resistance with research and development priorities. Twelve multi-drug-resistant organisms (MROs) are classified as ‘critical’, ‘high’ and ‘medium’ priority pathogens based on the severity of antibiotic resistance [30]. ESBL producing Enterobacteriaceae and carbapenem-resistant Enterobacteriaceae are examples of multi-drug resistant bacteria in the ‘critical’ group of priority pathogens [30]. Unlike a bibliometric review of data in Bahrain, which reported mostly ‘high’ priority pathogens, 71% of the isolates in this study are ‘critical’ priority pathogens [31]. The misuse and over use of the third-generation cephalosporins especially ceftriaxone, and the challenges of hand hygiene practices in these hospitals may explain this level of critical priority pathogens [15, 16, 32–34]. Unfortunately, in low-income countries like Sierra Leone, most of the antibiotics available are sold or dispensed over the counter without a prescription. Therefore, there is an urgent need for public education and enforcement of regulations to protect these essential commodities. None of the isolates are in the high or medium priority pathogen list, but this is expected as the study focused mainly on CAUTI and many of the MRO in these groups are not typical urinary pathogens [30].
Among the isolates in the critical pathogen group, we identified a high burden of ESBL Enterobacteriaceae from the urine of patients with CAUTI. The high burden of ESBL Enterobacteriaceae is a persistent problem, as previous studies in Sierra Leone have reported a similar ESBL burden in patients with surgical site infections [35, 36]. As alluded to earlier, the inappropriate antibiotic use and the challenges of hand hygiene compliance and implementation reported in some facilities in Sierra Leone may underlie the spread of ESBL Enterobacteriaceae in our healthcare settings [15, 16, 32–34]. To address the long-term threats associated with the persistent burden of ESBL Enterobacteriaceae in our hospitals, we recommend the integration of surveillance of MRO to routine clinical care and strengthen IPC and AMS interventions [37].
In the rank order of bacteria isolated from this study, E. coli, K. pneumoniae and K. oxytoca ranked first, second and third, respectively. This is not unique to this study as studies in Uganda, Ethiopia and Sudan reported similar findings [23–25]. In contrast, P. aeruginosa or Enterococcus species are the predominant pathogen isolated from the urine of CAUTI patients in Italy and Thailand [28, 38]. The variations in bacteria isolated from CAUTI patients across continents may be due to differences in environment, colonizing pathogens, or IPC practices.
Similar to studies in South Korea and Nigeria, Gram-negative bacteria isolates showed high resistance rates to the commonly available ampicillin, tetracycline and trimethoprim-sulfamethoxazole, but increase susceptibility to nitrofurantoin, piperacillin-tazobactam, amikacin and carbapenems [39, 40].
Our study is not bereft of limitations. The duration of catheterization was only considered during the hospital stay prior to the diagnosis of CAUTI. The duration of catheterization before admission and after collection of urine samples were not considered. This may have underestimated the average duration of catheter use. Although the study was conducted in two different geographic settings, we cannot generalize its findings to Sierra Leone as it was conducted only at the tertiary care level. Despite this, our study has provided the first evidence on the incidence of CAUTI in Sierra Leone.
Conclusion
In conclusion, this study reports high rates of catheterization and CAUTI in two tertiary hospitals in Sierra Leone, most of which are associated with multidrug-resistant bacteria.
We recommend urgent action to strengthen microbiological laboratory services, integrate antibiotic-resistant surveillance into routine clinical care, and establish functional antibiotic stewardship systems. In addition, healthcare facilities in Sierra Leone and similar countries across the world should develop and implement catheter bundles that provide clear guidance for catheter insertion, care and removal.
Acknowledgements
We appreciate the support provided by the administrations of the hospitals where the study was conducted. We are grateful to the research team members and acknowledge the cooperation of the ward staff, patients and patients’ relatives. We acknowledge the support provided by the Chinese Medical Expert Group at the 34 Military Hospital. We acknowledge the cooperation of the staff and patients and patients’ relatives at the hospitals where the study was conducted.
Abbreviations
- AMR
Antimicrobial Resistance
- CAUTI
Catheter-associated Urinary Tract Infection
- CRE
Carbapenem resistant Enterobacteriaceae
- ESBL
Extended-spectrum β-lactamase
- HAI
Healthcare-associated infections
- ICU
Intensive Care Units
- IPC
Infection Prevention and Control
- MRO
Multidrug-resistant organisms
- WHO
World Health Organization
Authors’ contributions
Conceptualization: S.L., J.B.W.R., S.K.C., and J.S.K. Methodology: S.L., U.B., E.F., L.Y., X.G., D.F.J., and G.F.D. Formal analysis: E.F., and S.L. Data curation: J.Z., Y.Z., S.S., L. Y. and U.B. Supervision: G.F.D., G.A.Y., S.S. and J.B.W.R. Resources: X.G., P.L., and L.Y. Funding: J.C.O. and X.G. Writing-original draft preparation: S.L., E.F., C.E.E.W., U.B., M.N.K., and Y.G. Writing-review and editing: G.A.Y, S.L., O.A., X.G., J.C.O., and E.F. All other authors do not have any conflict of interest. All authors read and approved the final manuscript.
Funding
This work was supported by the Structured Operational Research and Training Initiative (SORT IT), a global partnership coordinated by TDR, the Special Programme for Research and Training in Tropical Diseases at the World Health Organization; the Chinese Medical Expert Group at the 34 Military Hospital in Freetown, Sierra Leone.
Data availability
The data supporting this article is available in the repository of University of Sierra Leone and will be made available on request to the corresponding authors when required.
Declarations
Ethics approval and consent to participate
The protocol was approved by the Sierra Leone Ethics and Scientific Review Committee of the Ministry of Health and Sanitation, Government of Sierra Leone, in accordance with the relevant guidelines and regulations and the Declaration of Helsinki. Before participating in the study, each participant gave written informed consent. If patients were illiterate, the study and consent forms were explained verbally to them and consent was given by legal guardian fingerprinting. Participants who refused to consent were excluded from the study, but this did not affect their management.
Consent for publication
Not applicable.
Competing interests
E.F. receives his salary from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement (No 801076), through SSPH+ Global PhD Fellowship Programme in Public Health Sciences (GlobalP3HS). G.A.Y. reports salary support from the National Institutes of Health/AIDS Clinical Trials Group under Award Numbers 5UM1AI068636-15, 5UM1AI069501-09 and AI068636(150GYD212), and consultancy fees from Pfizer. All other authors do not have any conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sulaiman Lakoh and Le Yi contributed equally to this work and should be regarded as co-first authors.
Contributor Information
Sulaiman Lakoh, Email: lakoh2009@gmail.com.
Xuejun Guo, Email: xuejung@yahoo.com.
References
- 1.WHO. 2011: Report on the Burden of Endemic Health Care-Associated Infection Worldwide. Available at: https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf. Accessed on: May 1, 2022.
- 2.Ali S, Birhane M, Bekele S et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect Control. 2018; 7:2. Published 2018 Jan 5. 10.1186/s13756-017-0298-5. [DOI] [PMC free article] [PubMed]
- 3.Clarke K, Hall CL, Wiley Z, Tejedor SC, Kim JS, Reif L, Witt L, Jacob JT. Catheter-Associated urinary tract Infections in adults: diagnosis, treatment, and Prevention. J Hosp Med. 2020;15(9):552–6. doi: 10.12788/jhm.3292. [DOI] [PubMed] [Google Scholar]
- 4.Shuman EK, Chenoweth CE. Urinary catheter-Associated Infections. Infect Dis Clin North Am. 2018;32(4):885–97. doi: 10.1016/j.idc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Caramujo N, Carvalho M, Caria H. Prevalência Da algaliação sem indicação: um factor de risco evitável [Prevalence of inappropriate urinary catheterization: a preventable risk factor] Acta Med Port. 2011;24 Suppl 2:517–22. [PubMed] [Google Scholar]
- 6.Fukuoka K, Furuichi M, Ito K, Morikawa Y, Watanabe I, Shimizu N, Horikoshi Y. Longer duration of urinary catheterization increases catheter-Associated urinary tract Infection in PICU. Pediatr Crit Care Med. 2018;19(10):e547–50. doi: 10.1097/PCC.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 7.Laan BJ, Vos MC, Maaskant JM, van Berge Henegouwen MI, Geerlings SE. Prevalence and risk factors of inappropriate use of intravenous and urinary catheters in surgical and medical patients. J Hosp Infect. 2020;105(4):698–704. doi: 10.1016/j.jhin.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Luzum M, Sebolt J, Chopra V. Catheter-Associated urinary tract Infection, Clostridioides Difficile Colitis, Central Line-Associated Bloodstream Infection, and Methicillin-Resistant Staphylococcus aureus. Med Clin North Am. 2020;104(4):663–79. doi: 10.1016/j.mcna.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Lakoh S, Li L, Sevalie S, Guo X, Adekanmbi O, Yang G, Adebayo O, Yi L, Coker JM, Wang S, Wang T, Sun W, Habib AG, Klein EY. Antibiotic resistance in patients with clinical features of healthcare-associated infections in an urban tertiary hospital in Sierra Leone: a cross-sectional study. Antimicrob Resist Infect Control. 2020;9(1):38. 10.1186/s13756-020-0701-5. PMID: 32087751. [DOI] [PMC free article] [PubMed]
- 10.Kariuki S, et al. Antimicrobial resistance surveillance in Africa: successes, gaps and a roadmap for the future. Afr J Lab Med vol. 2018;7. 10.4102/ajlm.v7i2.924. 2 924. 6 Dec. [DOI] [PMC free article] [PubMed]
- 11.Pittet D, Allegranzi B, Storr J, Donaldson L. Clean Care is Safer Care’: the Global Patient Safety Challenge 2005–2006. Int J Infect Dis. 2006;10(6):419–24. doi: 10.1016/j.ijid.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Anderson DM, Cronk R, Fejfar D, Pak E, Cawley M, Bartram J. Safe Healthcare Facilities: a systematic review on the costs of establishing and Maintaining Environmental Health in Facilities in low- and Middle-Income Countries. Int J Environ Res Public Health. 2021;18(2):817. doi: 10.3390/ijerph18020817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health and Sanitation. Government of Sierra Leone Ebola Viral Disease Situation Report. [accessed on July 5, 2022]; Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/Ebola-Situation-Report_Vol-260.pdf.
- 14.GoSL 2016: National Infection Prevention and Control Policy. [accessed on July 2, 2022]. Available online: www.afro.who.int/publications/national-infection-prevention-and-control-guidelines-2016.
- 15.Lakoh S, Maruta A, Kallon C, Deen GF, Russell JBW, Fofanah BD, Kamara IF, Kanu JS, Kamara D, Molleh B, Adekanmbi O, Tavernor S, Guth J, Sagili KD, Wilkinson E. How Well Are Hand Hygiene Practices and Promotion Implemented in Sierra Leone? A cross-sectional study in 13 public hospitals. Int J Environ Res Public Health. 2022;19(7):3787. doi: 10.3390/ijerph19073787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakoh S, Firima E, Williams CEE, Conteh SK, Jalloh MB, Sheku MG, Adekanmbi O, Sevalie S, Kamara SA, Kamara MAS, Barrie U, Kamara GN, Yi L, Guo X, Haffner C, Kamara MN, Jiba DF, Namanaga ES, Maruta A, Kallon C, Kanu JS, Deen GF, Samai M, Okeibunor JC, Russell JBW. An Intra-COVID-19 Assessment of Hand Hygiene Facility, Policy and Staff Compliance in two hospitals in Sierra Leone: is there a difference between Regional and Capital City hospitals? Trop Med Infect Dis. 2021;6(4):204. doi: 10.3390/tropicalmed6040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fofanah BD, Abrahamyan A, Maruta A, Kallon C, Thekkur P, Kamara IF, Njuguna CK, Squire JS, Kanu JS, Bah AJ, Lakoh S, Kamara D, Hermans V, Zachariah R. Achieving Minimum standards for Infection Prevention and Control in Sierra Leone: Urgent need for a Quantum Leap in Progress in the COVID-19 era! Int J Environ Res Public Health. 2022;19(9):5642. doi: 10.3390/ijerph19095642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SSL. 2015: Sierra Leone Population Census. [(accessed on 14 August 2022)]. Available online: https://www.statistics.sl/images/StatisticsSL/Documents/Census/2015/sl_2015_phc_thematic_report_on_pop_structure_and_pop_distribution.pdf.
- 19.MICS, SIERRA LEONE MULTIPLE INDICATOR CLUSTER SURVEY. 2017:. 2017. Available online: https://www.statistics.sl/images/StatisticsSL/Documents/sierra_leone_mics6_2017_report.pdf (accessed on 09 August 2022).
- 20.Plachouras D, Lepape A, Suetens C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units [published correction appears in Intensive Care Med. 2018 Sep 17] Intensive Care Med 2018;44(12):2216–2218. 10.1007/s00134-018-5113-0. [DOI] [PMC free article] [PubMed]
- 21.CDC/NHSN. Surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting. 2013; Available at http://www.socinorte.com/wp-content/uploads/2013/03/Criterios-de-IN-2013.pdf. [DOI] [PubMed]
- 22.Clinical and Laboratory Standards Institute (January, M11. 2020 update). Performance standards for antimicrobial susceptibility testing: CLSI publication M02, M07, and. Available at: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf.
- 23.Oumer Y, Regasa Dadi B, Seid M, Biresaw G, Manilal A. Catheter-Associated urinary tract Infection: incidence, Associated factors and drug resistance patterns of bacterial isolates in Southern Ethiopia. Infect Drug Resist. 2021;14:2883–94. doi: 10.2147/IDR.S311229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musinguzi B, Kabajulizi I, Mpeirwe M, Turugurwa J, Kabanda T. Incidence and etiology of catheter associated urinary tract Infection among admitted patients at Kabale Regional Referral Hospital, South Western Uganda. Adv Infect Dis. 2019;09(03):183–96. doi: 10.4236/aid.2019.93014. [DOI] [Google Scholar]
- 25.Ahmed M. Pattern of nosocomial urinary tract Infections among Sudanese patients. Br Microbiol Res J. 2012;2(2):53–61. doi: 10.9734/BMRJ/2012/1255. [DOI] [Google Scholar]
- 26.Letica-Kriegel AS, Salmasian H, Vawdrey DK, Youngerman BE, Green RA, Furuya EY. Identifying the risk factors for catheter-associated urinary tract Infections: a large cross-sectional study of six hospitals. BMJ Open. 2019;9:1–7. doi: 10.1136/bmjopen-2018-022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner A, Mitchell B, Beckingham W, Fasugba O. A point prevalence cross-sectional study of healthcare-associated urinary tract Infections in six Australian hospitals. BMJ Open. 2014;4(7):1–9. doi: 10.1136/bmjopen-2014-005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbadoro P, Labricciosa FM, Recanatini C, Gori G, Tirabassi F, Martini E. Catheter-associated urinary tract Infection: role of the setting of catheter insertion. Am J Infect Control. 2015;43(7):707–10. doi: 10.1016/j.ajic.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Meddings J, Saint S, Krein SL, Gaies E, Reichert H, Hickner A, McNamara S, Mann JD, Mody L. Systematic Review of Interventions to Reduce Urinary Tract Infection in Nursing Home Residents. J Hosp Med. 2017;12(5):356–368. 10.12788/jhm.2724. PMID: 28459908. [DOI] [PMC free article] [PubMed]
- 30.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and Tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 31.Asokan GV, Ramadhan T, Ahmed E, Sanad H. WHO Global Priority pathogens list: a bibliometric analysis of Medline-PubMed for Knowledge mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med J. 2019;34(3):184–93. doi: 10.5001/omj.2019.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakoh S, et al. High levels of surgical antibiotic prophylaxis: implications for hospital-based antibiotic stewardship in Sierra Leone. Antimicrobial Stewardship & Healthcare Epidemiology; 2022. 10.1017/ash.2022.252. [DOI] [PMC free article] [PubMed]
- 33.Lakoh S, Adekanmbi O, Jiba DF, Deen GF, Gashau W, Sevalie S, Klein EY. Antibiotic use among hospitalized adult patients in a setting with limited laboratory infrastructure in Freetown Sierra Leone, 2017–2018. Int J Infect Dis. 2020;90:71–6. doi: 10.1016/j.ijid.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Lakoh S, John-Cole V, Luke RDC, Bell N, Russell JBW, Mustapha A, Barrie U, Abiri OT, Coker JM, Kamara MN, Coker FJ, Adekanmbi O, Kamara IF, Fofanah BD, Jiba DF, Adeniji AO, Kenneh S, Deen GF, Moon TD, Yendewa GA, Firima E. Antibiotic use and consumption in Freetown, Sierra Leone: a baseline report of prescription stewardship in outpatient clinics of three tertiary hospitals. IJID Reg. 2023;7:43–51. doi: 10.1016/j.ijregi.2023.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakoh S, Yi L, Sevalie S, Guo X, Adekanmbi O, Smalle IO, Williams N, Barrie U, Koroma C, Zhao Y, Kamara MN, Cummings-John C, Jiba DF, Namanaga ES, Deen B, Zhang J, Maruta A, Kallon C, Liu P, Wurie HR, Kanu JS, Deen GF, Samai M, Sahr F, Firima E. Incidence and risk factors of surgical site Infections and related antibiotic resistance in Freetown, Sierra Leone: a prospective cohort study. Antimicrob Resist Infect Control. 2022;11(1):39. doi: 10.1186/s13756-022-01078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakoh S, Yi L, Russell JBW, Zhang J, Sevalie S, Zhao Y, Kanu JS, Liu P, Conteh SK, Williams CEE, Barrie U, Sheku MG, Jalloh MB, Adekanmbi O, Jiba DF, Kamara MN, Deen GF, Okeibunor JC, Yendewa GA, Guo X, Firima E. The burden of surgical site Infections and related antibiotic resistance in two geographic regions of Sierra Leone: a prospective study. Ther Adv Infect Dis. 2022;9:20499361221135128. doi: 10.1177/20499361221135128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakoh S, Bawoh M, Lewis H, Jalloh I, Thomas C, Barlatt S, Jalloh A, Deen GF, Russell JBW, Kabba MS, Batema MNP, Borgstein C, Sesay N, Sesay D, Nagi NK, Firima E, Thomas S. Establishing an antimicrobial stewardship program in Sierra Leone: a report of the experience of a low-income country in West Africa. Antibiot (Basel) 2023;12(3):424. doi: 10.3390/antibiotics12030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotikula I, Chaiwarith R. Epidemiology of catheter-associated urinary tract Infections at Maharaj Nakorn Chiang Mai hospital, northern Thailand. Southeast Asian J Trop Med Public Health. 2018;49(1):113–22. [Google Scholar]
- 39.Park JJ, Seo YB, Kim SR, Park HJ, Eom JS, Yoo H. Incidence of catheter-associated urinary tract Infection in hospitals with less than 300 beds. Korean J Healthc Assoc Infect Control Prev. 2019;24(1):11–8. doi: 10.14192/kjhaicp.2019.24.1.11. [DOI] [Google Scholar]
- 40.Taiwo S, Aderounmu A. Catheter associated urinary tract Infection: aetiologic agents and antimicrobial susceptibility pattern in Ladoke Akintola University Teaching Hospital, Osogbo, Nigeria. Afri J Biomed Res. 2009;9(3):141–8. doi: 10.4314/ajbr.v9i3.48897. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this article is available in the repository of University of Sierra Leone and will be made available on request to the corresponding authors when required.