Figure 3.
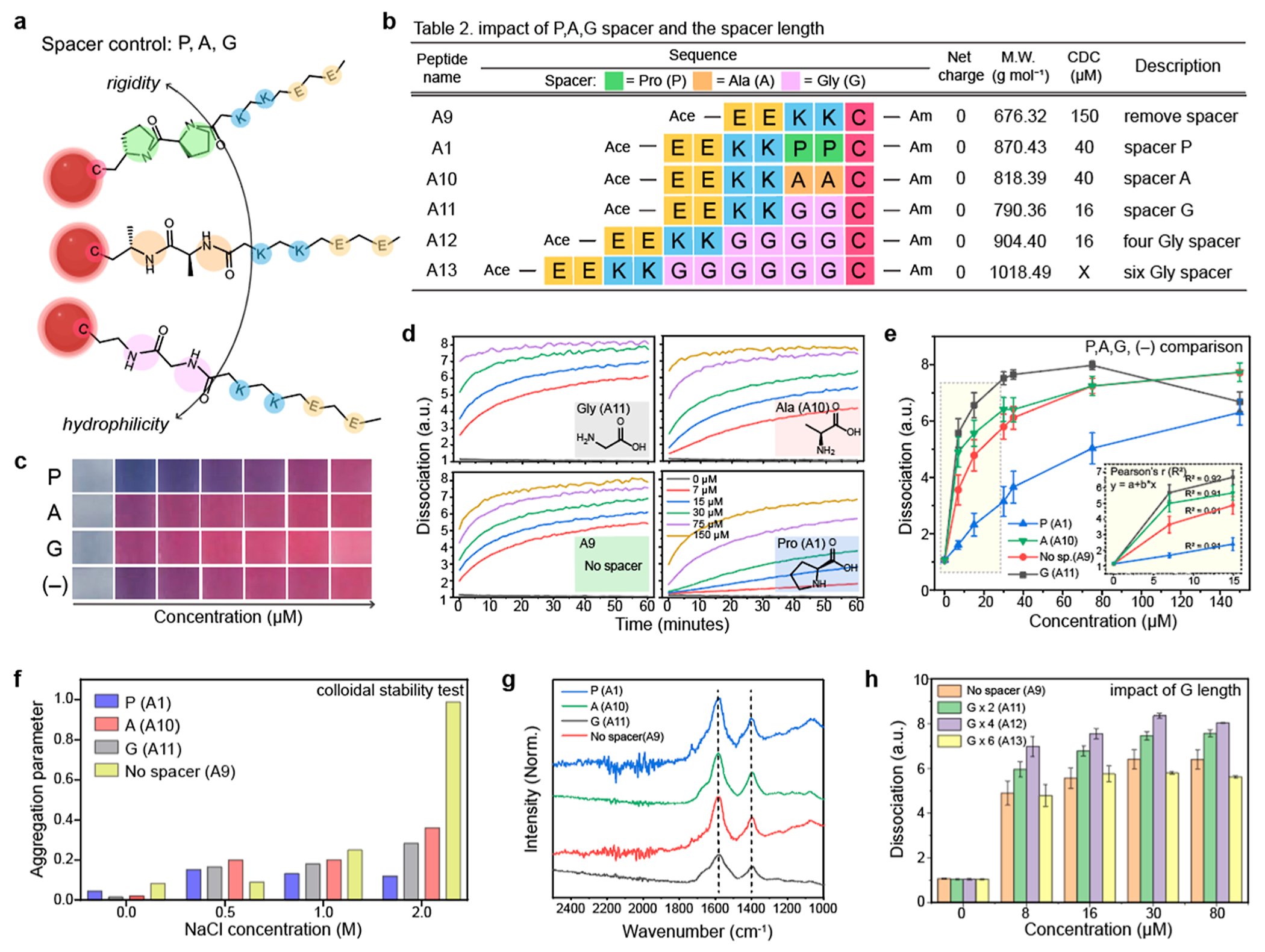
Impact of hydrophilicity and steric bulk on particle dissociation. (a) Different Pro-, Ala-, and Gly-spacers have different nature of rigidity and hydrophilicity which can impact on the dissociation capacity. Table 2 in (b) describes peptide sequences that are designed to investigate the impact of spacers. (c) Photographs of the dissociated AuNPs by the PP, AA, and GG spacers and without spacer (−) as a negative control. (d) Time-dependent particle dissociations driven by the A1, A9, A10, and A11 peptides, respectively. (e) Gly spacer showed a higher dissociation capacity than the Pro- and Ala- spacers. (f) Aggregation parameter of the dissociated AuNPs driven by the A1, A9, A10, and A11 peptides. The results showed that the peptide with spacer can provide higher colloidal stability for AuNPs than the peptide without spacer. (g) FTIR data of the dissociated AuNPs by the A1, A9, A10, and A11 peptides. The peaks at 1400 and 1600 cm−1 were attributed to the carboxyl group in the Glu amino acid. (h) Impact of the spacer length on the particle dissociation. Increasing the length of the spacer (from two to four) improved dissociation capacity, while the spacer with six Glu (i.e., A13) showed lower dissociation capacity than the A12 peptide. The panel (e,f,h) repeated three independent times and showed similar results.
