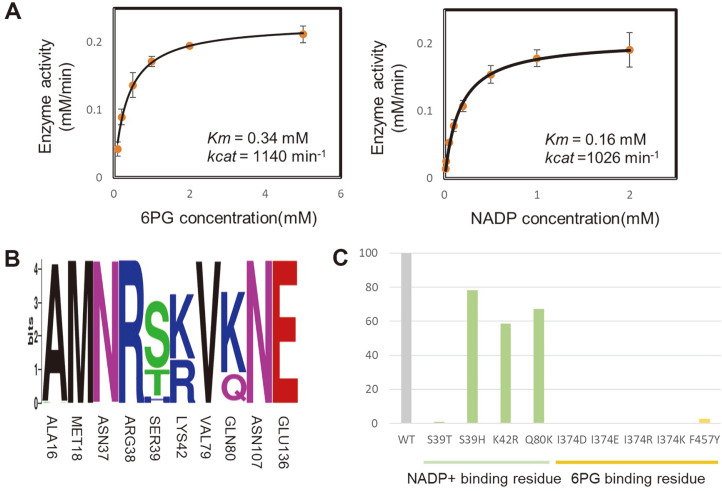
Fig. 5. Enzymatic kinetics of Cg6PGD.
(A) Kinetic analysis of Cg6PGD. The reaction velocity was plotted vs. the substrate (6PG) concentration (left) and co-factor (NADP) concentration (right) based on the Michaelis-Menten equation. The experiments were performed in duplicate and the standard deviation is indicated by the error bar. Various concentrations of 6PG (0.1~5 mM) and NADP (0.01~2 mM) were used. (B) Conservation analysis of reisudes associated with NADP-binding. (C) Relative activity of Cg6PGD. The activity value of the mutants is expressed with the wild-type form set at 100%. The green and yellow graphs present the relative activities of the NADP-binding residue and 6PG-binding residue, respectively.