Abstract
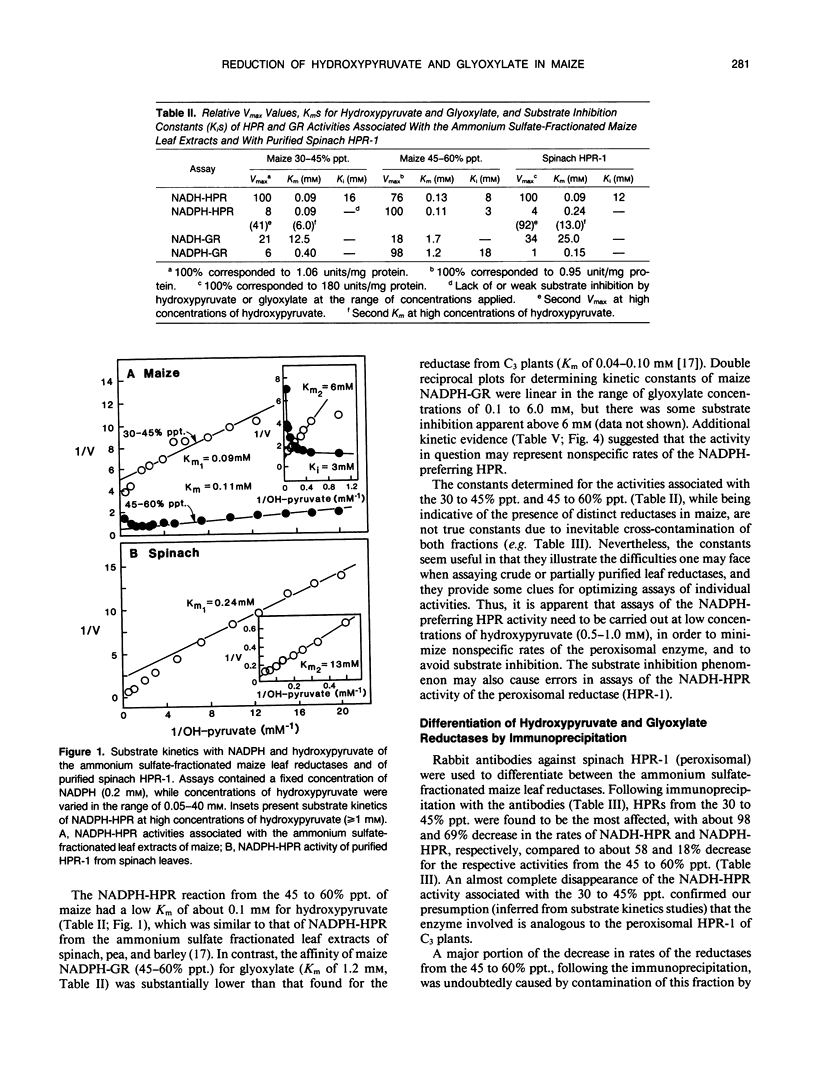
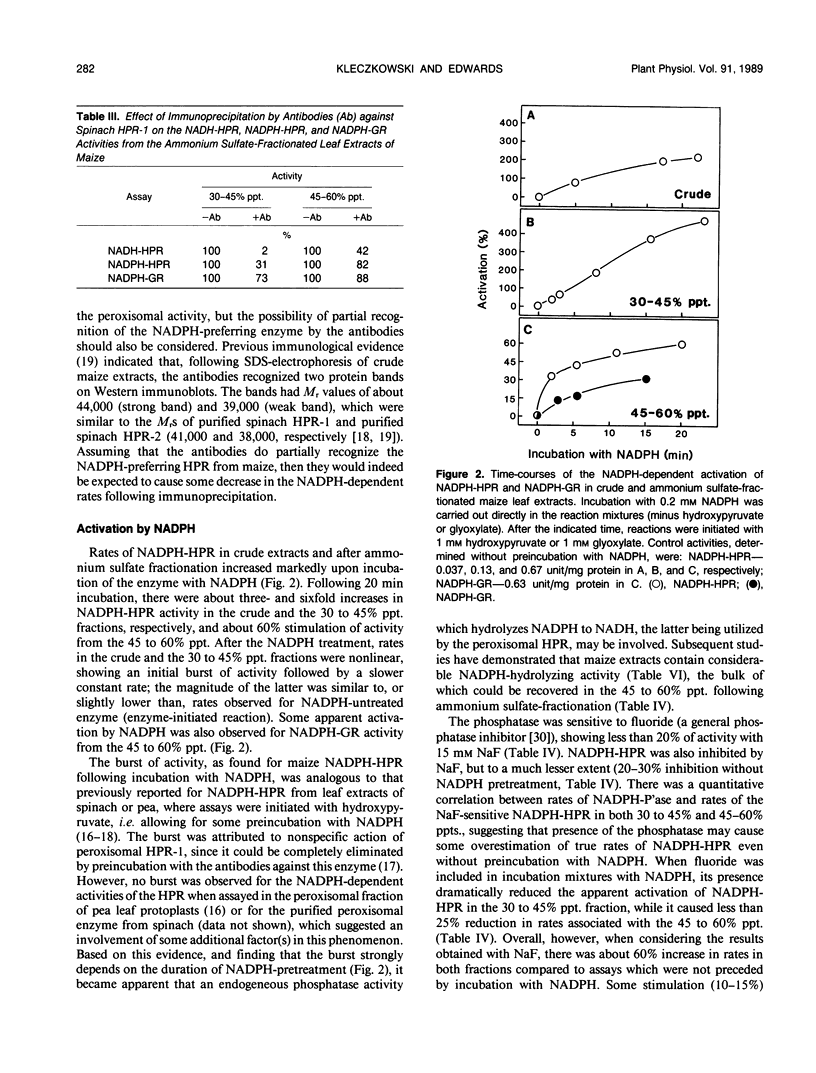
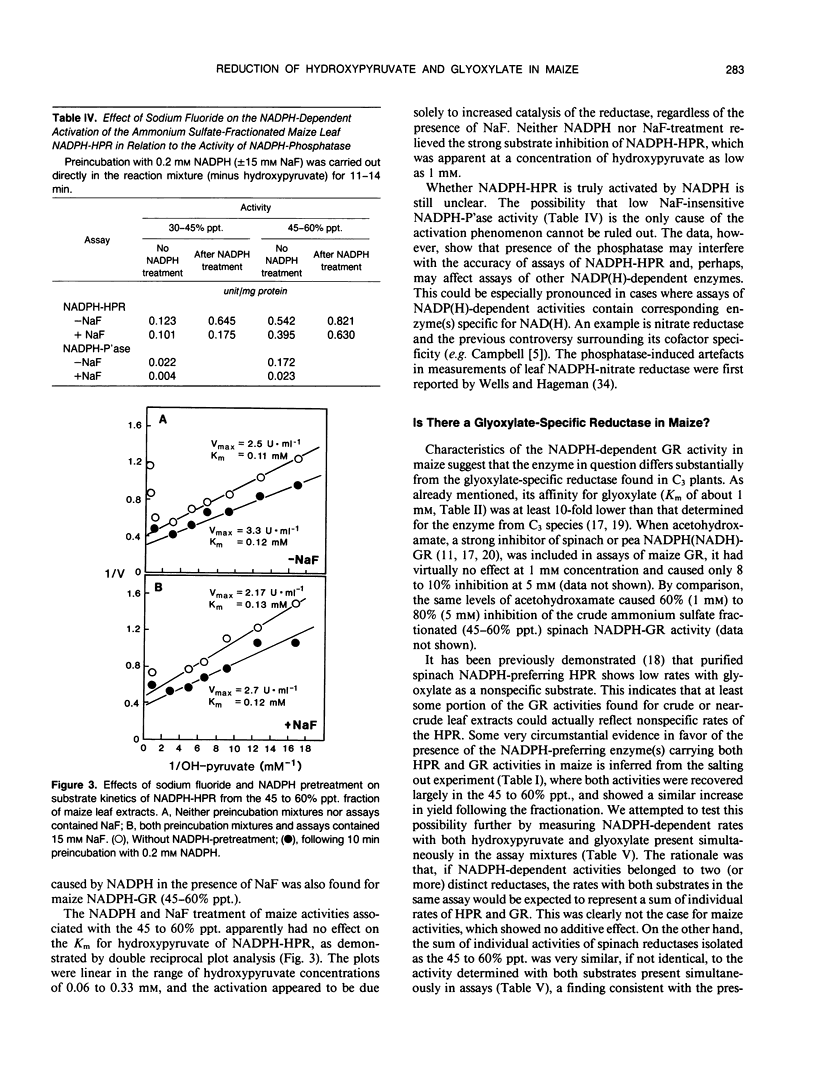
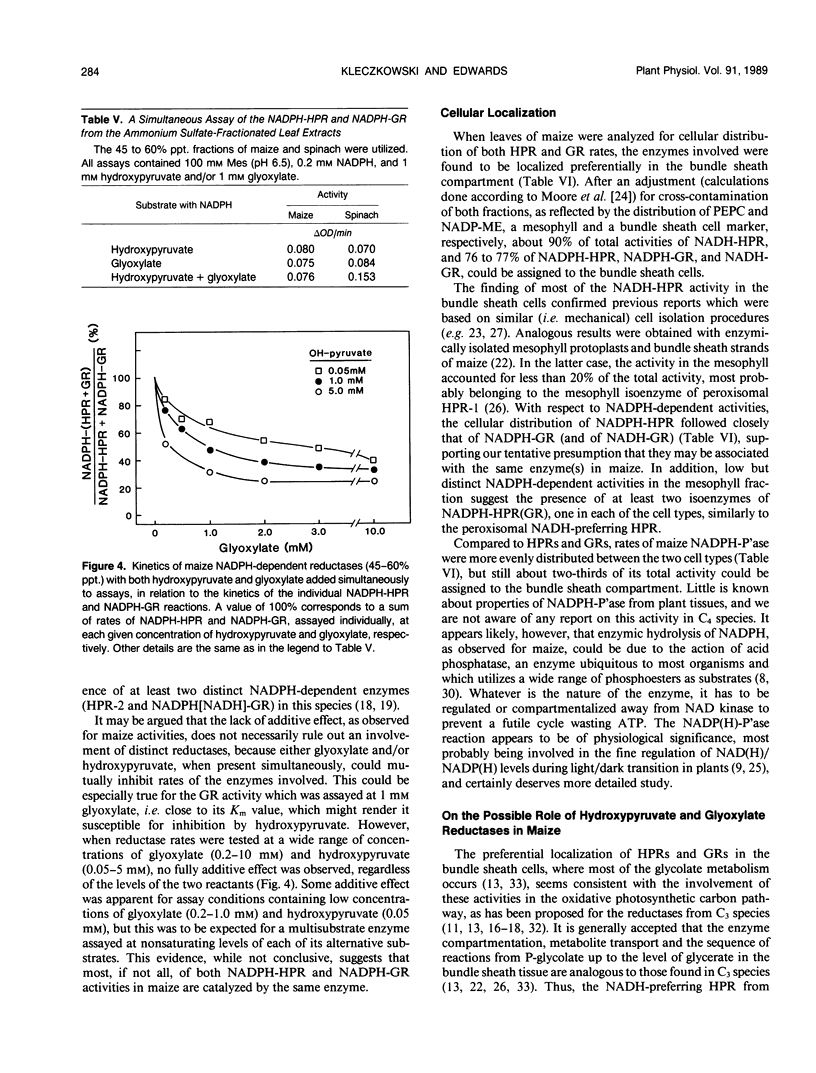
At least two hydroxypyruvate reductases (HPRs), differing in specificity for NAD(P)H and (presumably) utilizing glyoxylate as a secondary substrate, were identified by fractionation of crude maize leaf extracts with ammonium sulfate. The NADH-preferring enzyme, which most probably represented peroxisomal HPR, was precipitated by 30 to 45% saturated ammonium sulfate, while most of the NADPH-dependent activity was found in a 45 to 60% precipitate. The HPRs had similar low Kms for hydroxypyruvate (about 0.1 millimolar), regardless of cofactor, while affinities of glyoxylate reductase (GR) reactions for glyoxylate varied widely (Kms of 0.4-12 millimolar) depending on cofactor. At high hydroxypyruvate concentrations, the NADPH-HPR from the 30 to 45% precipitate showed negative cooperativity with respect to this reactant, having a second Km of 6 millimolar. In contrast, NADPH-HPR from the 45 to 60% precipitate was inhibited at high hydroxypyruvate concentrations (K1 of 3 millimolar) and, together with NADPH-GR, had only few, if any, common antigenic determinants with NADH-HPR from the 30 to 45% fraction. Both NADPH-HPR and NADPH-GR activities from the 45 to 60% precipitate were probably carried out by the same enzyme(s), as found by kinetic studies. Following preincubation with NADPH, there was a marked increase (up to sixfold) in activity of NADPH-HPR from either crude or fractionated extracts. Most of this increase could be attributed to an artefact resulting from an interference by endogeneous NADPH-phosphatase, which hydrolyzed NADPH to NADH, the latter being utilized by the NADH-dependent HPR. However, in the presence of 15 millimolar fluoride (phosphatase inhibitor), preincubation with NADPH still resulted in over 60% activation of NADPH-HPR. The NADPH treatment stimulated the Vmax of the reductase but had no effect on its Km for hydroxypyruvate. Enzyme distribution studies revealed that both NADH and NADPH-dependent HPR and GR activities were predominantly localized in the bundle sheath compartment. Rates of NADPH-HPR and NADPH-GR in this tissue (over 100 micromoles per hour per milligram of chlorophyll each) are in the upper range of values reported for leaves of C3 species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chapman D. J., Leech R. M. Phosphoserine as an early product of photosynthesis in isolated chloroplasts and in leaves of Zea mays seedlings. FEBS Lett. 1976 Oct 1;68(2):160–164. doi: 10.1016/0014-5793(76)80427-9. [DOI] [PubMed] [Google Scholar]
- Dry I. B., Dimitriadis E., Ward A. D., Wiskich J. T. The photorespiratory hydrogen shuttle. Synthesis of phthalonic acid and its use in the characterization of the malate/aspartate shuttle in pea (Pisum sativum) leaf mitochondria. Biochem J. 1987 Aug 1;245(3):669–675. doi: 10.1042/bj2450669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTI G., TOGNOLI C., PARISI B. Purification from pea leaves of a phosphatase that attacks nucleotides. Biochim Biophys Acta. 1962 Aug 13;62:251–260. doi: 10.1016/0006-3002(62)90038-0. [DOI] [PubMed] [Google Scholar]
- Fu C. F., Gibbs M. Effects of temperature pretreatment in the dark on photosynthesis of the intact spinach chloroplast. Plant Physiol. 1988 Sep;88(1):207–212. doi: 10.1104/pp.88.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husic D. W., Tolbert N. E. NADH:hydroxypyruvate reductase and NADPH:glyoxylate reductase in algae: partial purification and characterization from Chlamydomonas reinhardtii. Arch Biochem Biophys. 1987 Feb 1;252(2):396–408. doi: 10.1016/0003-9861(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Kleczkowski L. A., Givan C. V., Hodgson J. M., Randall D. D. Subcellular Location of NADPH-Dependent Hydroxypyruvate Reductase Activity in Leaf Protoplasts of Pisum sativum L. and Its Role in Photorespiratory Metabolism. Plant Physiol. 1988 Dec;88(4):1182–1185. doi: 10.1104/pp.88.4.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D., Blevins D. G. Inhibition of Spinach Leaf NADPH(NADH)-Glyoxylate Reductase by Acetohydroxamate, Aminooxyacetate, and Glycidate. Plant Physiol. 1987 Jul;84(3):619–623. doi: 10.1104/pp.84.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D., Blevins D. G. Purification and characterization of a novel NADPH(NADH)-dependent glyoxylate reductase from spinach leaves. Comparison of immunological properties of leaf glyoxylate reductase and hydroxypyruvate reductase. Biochem J. 1986 Nov 1;239(3):653–659. doi: 10.1042/bj2390653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D. Purification and characterization of a novel NADPH(NADH)-dependent hydroxypyruvate reductase from spinach leaves. Comparison of immunological properties of leaf hydroxypyruvate reductases. Biochem J. 1988 Feb 15;250(1):145–152. doi: 10.1042/bj2500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D., Warren W. A. The kinetic properties of spinach leaf glyoxylic acid reductase. J Biol Chem. 1970 Aug 10;245(15):3831–3839. [PubMed] [Google Scholar]
- Liu A. Y., Black C. C., Jr Glycolate metabolism in mesophyll cells and bundle sheath cells isolated from crabgrass, Digitaria sanguinalis (L.) Scop., leaves. Arch Biochem Biophys. 1972 Mar;149(1):269–280. doi: 10.1016/0003-9861(72)90322-0. [DOI] [PubMed] [Google Scholar]
- Muto S., Miyachi S. Light-induced conversion of nicotinamide adenine dinucleotide to nicotinamide adenine dinucleotide phosphate in higher plant leaves. Plant Physiol. 1981 Aug;68(2):324–328. doi: 10.1104/pp.68.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. R., Edwards G. E. Provisions of reductant for the hydroxypyruvate to glycerate conversion in leaf peroxisomes : a critical evaluation of the proposed malate/aspartate shuttle. Plant Physiol. 1983 Jul;72(3):728–734. doi: 10.1104/pp.72.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. G. Acid phosphatase from tobacco leaves. Arch Biochem Biophys. 1966 Oct;117(1):1–9. doi: 10.1016/0003-9861(66)90118-4. [DOI] [PubMed] [Google Scholar]
- Titus D. E., Hondred D., Becker W. M. Purification and characterization of hydroxypyruvate reductase from cucumber cotyledons. Plant Physiol. 1983 Jun;72(2):402–408. doi: 10.1104/pp.72.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Usuda H., Edwards G. E. Localization of glycerate kinase and some enzymes for sucrose synthesis in c(3) and c(4) plants. Plant Physiol. 1980 May;65(5):1017–1022. doi: 10.1104/pp.65.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. N., Hageman R. H. Specificity for nicotinamide adenine dinucleotide by nitrate reductase from leaves. Plant Physiol. 1974 Aug;54(2):136–141. doi: 10.1104/pp.54.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Alternate pathways of glycolate synthesis in tobacco and maize leaves in relation to rates of photorespiration. Plant Physiol. 1973 Feb;51(2):299–305. doi: 10.1104/pp.51.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]


