Abstract
The interactions between polymers and the immune system remains poorly controlled. In some instances, the immune system can produce antibodies specific to polymer constituents. Indeed, roughly half of pegloticase patients without immunomodulation develop high titers of anti-PEG antibodies (APA) to the PEG polymers on pegloticase, which then quickly clear the drug from circulation and render the gout treatment ineffective. Here, using pegloticase as a model drug, we show that addition of high molecular weight (MW) free (unconjugated) PEG to pegloticase allows us to control the immunogenicity and mitigates APA induction in mice. Compared to pegloticase mixed with saline, mice repeatedly dosed with pegloticase containing different MW or amount of free PEG possessed 4- to 12- fold lower anti-PEG IgG, and 6- to 10- fold lower anti-PEG IgM, after 3 rounds of pegloticase dosed every 2 weeks. The markedly reduced APA levels, together with competitive inhibition by free PEG, restored the prolonged circulation of pegloticase to levels observed in APA-naïve animals. In contrast, mice with pegloticase-induced APA eliminated nearly all pegloticase from the circulation within just four hours post-injection. These results support the growing literature demonstrating free PEG may effectively suppress drug-induced APA, which in turn may offer sustained therapeutic benefits without requiring broad immunomodulation. We also showed free PEG effectively blocked the PEGylated protein from binding with cells expressing PEG-specific B cell receptors. It provides a template of how we may be able to tune the interactions and immunogenicity of other polymer-modified therapeutics.
Keywords: PEG, Anti-PEG antibody, Immune reaction, Polymer, Pharmacokinetics
Graphical abstract
1. Introduction
Polyethylene glycol (PEG), due to its ability to resist protein adsorption, limit immuno-stimulation, and reduce clearance by the reticuloendothelial system, has been widely used to extend the circulation times of protein and nanoparticle therapeutics [1–4]. Unfortunately, recent animal and human studies have shown that anti-PEG antibodies (APA) can be induced by select PEGylated therapeutics [5–8]. Furthermore, while the majority of the general population possess low titers of pre- existing APA, a small fraction can possess substantial titers, comparable to levels seen in patients with drug-induced PEG immunity [9]. The high prevalence of individuals with detectible levels of APA implies the presence of immune memory against PEG that could lead to rapid APA induction in these individuals. Regardless of whether APA is pre-existing or acutely induced, high titers of APA in serum can quickly bind to PEGylated drugs in circulation [10, 11]. This results in rapid hepatic clearance that greatly shortens the circulation kinetics of the PEGylated drugs, termed accelerated blood clearance (ABC), and renders the drugs non-efficacious and potentially less safe [11, 12]. In light of the increasing number of PEGylated therapeutics that are either FDA approved or in clinical development, there is an urgent need to develop interventions that can limit APA induction and restore the safe and efficacious use of PEGylated drugs in patients at risk of developing high APA titers.
Gout is a debilitating and painful inflammatory arthritis, characterized by high levels of uric acid in the serum and the deposition of uric acid crystals in the joints. An obvious therapeutic strategy in the management of gout is to reduce serum uric acid level that in turn may decrease urate deposits and alleviate clinical symptoms. In patients with uncontrolled gout, sustaining high uricase levels thus offers the potential to address the root cause of the disease. KRYSTEXXA® (pegloticase), a PEG-conjugated enzyme that degrades uric acid to allantoin, is currently the only FDA-approved treatment for severe, treatment-refractory chronic gout that affects ~25,000 –100,000 patients in the U.S. each year [13]. Pegloticase is comprised of ~40 chains of 10 kDa PEG covalently bound to the tetrameric uricase; the PEGylation minimizes immunogenicity against the uricase, and prolongs circulation kinetics of the enzyme by reducing the rate of renal clearance due to the increased hydrodynamic diameter. Unfortunately, clinical studies have revealed that as high as 80–90% of pegloticase-treated patients without immunomodulation instead develop antibodies directed against the PEG chains [8, 14]. APA titer directly correlates to reduced serum uricase activity, and results in a rebound of serum uric acid levels often within weeks of initiating the treatment.
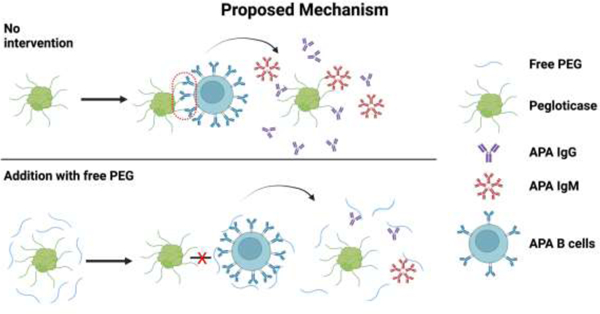
The two most common strategies to limit APA induction and APA-mediated accelerated blood clearance of PEGylated drugs involve either replacing PEG with alternative polymers [15–19], or utilizing broad immunosuppression to limit APA induction [20–22], such as the recent FDA approval of use of methotrexate (MTX) together with pegloticase [23, 24]. Our group has been pursuing a third strategy, namely relying on unconjugated (free) PEG molecules to serve as decoys that competitively inhibit serum APA from binding the PEGylated drug, as well as reduce PEGylated drug-mediated stimulation of APA+ B-cells, leading to lower levels of induced APA. We previously found that pre-infusion with a large dose of free PEG was highly effective at restoring prolonged pegloticase circulation in mice with already high titers of pegloticase-induced APA [25]. Nevertheless, there are concerns with chronic use of very large doses of PEG, and treatment burden created by a two-step infusion regimen. It is also far more attractive to prevent stimulation of APA by reducing the immunogenicity of the underlying drug, given the need for redosing. This led us to explore, in this work, whether a simple reformulation of pegloticase, based on addition of low doses of high MW free PEG to pegloticase, could sufficiently reduce PEG immunogenicity and limit APA induction, and in turn sustain prolonged circulation of pegloticase over multiple rounds of injection.
2. Materials & Methods
2.1. In vivo studies of pegloticase with and without co-administration of free PEG mixed prior to injection.
BALB/c mice were obtained from Charles Rivers Laboratories. All animal procedures in this study were approved by the University of North Carolina at Chapel Hill’s Institutional Animal Care and Use Committee. Clinical-grade Pegloticase was obtained from Horizon Therapeutics, and stored at 4°C until use. Pegloticase was diluted with different concentrations of sterile filtered (5 mg/kg or 50 mg/kg) of 40 kDa PEG (SERVA, 33139) or (5 mg/kg) 100 kDa PEG (Sigma, 181986–250G) prior to dosing to mice at 0.15 mg/kg pegloticase in 150μL saline. Pegloticase diluted with saline served as negative control (and are referred to as pegloticase-only in text). On Days 0, 14 and 28, mice were administrated with 150 μL of these solutions through the tail vein. On Days −1, 13, 27 and 41, 200 μL whole blood was collected by mandibular bleed. The blood samples were left in tubes with EDTA on ice until centrifuged at 2000g for 15 mins at 4 °C. Plasma was then collected and stored at −80°C for further analysis.
2.2. ELISA measurement of anti-PEG IgG and IgM.
Anti-PEG IgG and IgM were assessed via competition ELISA designed previously in the lab [5, 9]. Briefly, 50 μg/mL DSPE-PEG 5000 (NANOCS, PG1-DS-5K) was incubated in non-treated half-area 96-well plates (Corning, 3695) overnight at 4°C. The plates were washed three times with PBS, and blocked with 5% non-fat milk (Lab scientific bioKEMIX, M0841) for 1 hour at room temperature to reduce non-specific binding. Mouse anti-PEG IgG (Silver Lake, CH2076) and IgM (Academia Sinica, AGP4 (AGP4-PABM-A) standards were serial diluted in 1% milk to generate standard curves (Supp Figure S1). Samples were diluted 100-fold in 1% milk or in 1% milk with 10 mg/mL PEG 8 kDa (Sigma, P2139–500G), and measured in triplicate. Herein, large amount of PEG was added as competition setup to evaluate the specificity of binding (Supp Figure S2). The plates were kept at 4°C overnight, followed by six times washing with PBS. HRP conjugated goat anti-mouse IgG (Invitrogen, A28177, Lot#2596484) and goat anti-mouse IgM (Invitrogen, 62–6820, Lot#WB317635) were added to the wells. After one-hour incubation at room temperature and another round of washing, TMB (Thermo Fisher, 34029) was added to the plates, followed by 1N HCL ~10 mins later. The absorbance of plates was read at 450 and 595 nm, and absorbance value of each sample was calculated by subtracting absorbance of corresponding competition setup. A 5-parameter regression based on standard curve was applied to determine the concentrations of samples. Two independent experiments were performed for all samples, and the mean concentrations were reported.
2.3. PET/CT imaging.
[89Zr]-pegloticase was prepared in the BRIC radiochemistry core facility, following previous published protocol [26]. Briefly, pegloticase was first reacted with p-SCN-Bn-Deferoxamine via deferoxamine (DF) chelation, and pegloticase-chelator conjugate was purified with a PD-10 column. 89Zr was then conjugated on pegloticase-chelator conjugate and purified right before injection in vivo. On Day 42, all mice received [89Zr]-pegloticase. Naïve BALB/c mice (never exposed to any PEG product before) were included as the negative control, and were administrated with [89Zr]-pegloticase with saline. PET/CT imaging procedure closely followed previous published protocol with a small animal PET/CT imaging system (SuperArgus 4R, Sedecal Inc., Spain) [25]. The mice were anesthetized by isoflurane and placed in a multi-animal imaging cradle in groups of 4 for simultaneous imaging. Mice received a bolus injection of [89Zr]-pegloticase (4.0 ± 0.3 MBq, equivalent mass ~5.2 μg of pegloticase), followed immediately with a dynamic PET scan of 60 mins. CT was conducted afterwards for anatomical reference. Static PET/CT scans were conducted at 1, 4, 24, 48, 72, and 96 hours post injection of [89Zr]-pegloticase. For the first 1-hour dynamic scan, images were binned into 19 frames with the following scheme: 6×10s, 4×30s, 2×60s, 3×300s, and 4×600s. Image data were reconstructed with 3D-OSEM reconstruction algorithms with scatter, attenuation, and decay correction. After the PET scan at 96 hours, the mice were sacrificed. Blood was collected and organs including liver, lung, kidney, heart, and muscle tissue harvested for gamma counting. Blood samples were centrifuged to separate plasma from blood cells. Gamma counting was conducted using Wizzard2 Gamma Counter (PerkinElmer, Inc. USA). For image analysis, PET and CT images were first registered and ROIs were drawn in major organs from CT images and superimposed to PET images for ROI-based analysis. Analysis of PET/CT scans was performed via PMOD 4.0 software, and standardized uptake value (SUV) of each ROI was calculated. %ID/g was reported as the quotient of dividing signal concentration of each ROI by decay-corrected injection dose. In this study, we define the total signal of each mouse to be the weighted summary of heart and other major organs including liver, kidney, muscle and lung, as these organs were the most representative organs regarding drug biodistribution; notably, the activity excreted from the bladder was not included. The weighted fraction of each organ was determined by the estimated organ mass reported in previous empirical estimation [27–29].
2.4. Expression of anti-PEG B cell receptors (BCR) on cells.
We designed an expression plasmid encoding LRRC signal peptide, PEG-binding domain (m6.3 IgG), strep tag, CD28 transmembrane domain and CD79β intracellular domain, and transfected it into Expi293 cells (A14527, ThermoFisher) [30, 31]. Enhancers were added 22 hrs later following manufacturer’s protocol. Cells were harvested on Day 2 or Day 3 for further assays. Expression of anti-PEG BCR on cells were confirmed with anti-strep tag antibody-FITC conjugate (A00875–40, Genscript).
2.5. Assessing PEG and model PEGylated protein interactions with anti-PEG BCR+ cells.
BSA was PEGylated by one-hour incubation with FITC-PEG-NHS 3.4K (PG2-FCNS-3k, NANOCS) in PBS-EDTA buffer at room temperature, following published protocols [32]. Unconjugated PEG was removed by Zeba column (40 kDa cutoff, 87766, ThermoFisher). We then incubated 1 × 106 anti-PEG BCR expressing cells with 10ug of BSA-PEG-FITC, with or without 1mg of 10 kDa methoxy-PEG (732621, Sigma). Expi293 cells that were not transfected were used as negative control to identify binding due to anti-PEG BCR. Cells were then assessed by flow cytometry on Attune NxT (ThermoFisher, USA). Only living cells, gated with live-dead dye (L34964, ThermoFisher), were included for analysis.
2.6. Statistical analysis
All data was statistically analyzed with one-way ANOVA followed by Bonferroni-adjust for multiple comparisons, Student’s t-test, and nonparametric Spearman correlation using the GraphPad Prism software (GraphPad Software Inc., USA). Significance was considered as statistical analysis value less than 0.05 (p < 0.05) between groups. * denotes p < 0.05; ** denotes p < 0.01; *** denotes p < 0.001.
3. Results
3.1. Addition of free PEG to pegloticase suppresses APA induction in mice
Clinically, pegloticase is given intravenously every two weeks. To mimic this clinical scenario, we injected pegloticase intravenously, with or without free PEG added prior to injection, into immunocompetent BALB/c mice biweekly on Days 0, 14, and 28. We then quantified anti-PEG IgG and IgM levels in serum collected every two weeks starting from one day prior the first injection (Figure 1A). Consistent with our previous study [25], and in good agreement with clinical observations [33–35], appreciable APA titers were detected in the blood by 13 days after the first pegloticase injection, with ~14.8 and ~3.6 μg/mL of APA IgG and IgM, respectively. This further increased following the second and third injections, to ~58.0 and ~7.0 μg/mL of APA IgG and IgM, respectively by Day 41 (Figure 1B, 1C). In contrast, both APA IgG and IgM levels in mice injected with pegloticase containing different amounts and MW of free PEG were markedly reduced across the entire study. Nearly 2 weeks after the third injection, the average APA IgG levels were ~5.0, ~15.4 and ~12.0 μg/mL in mice receiving pegloticase together with 5 mg/kg 40 kDa PEG, 50 mg/kg 40 kDa PEG and 5 mg/kg 100 kDa PEG, respectively, or ~12-, ~4- and ~5-fold lower than the APA IgG levels in mice receiving pegloticase alone. Similarly, the inclusion of free PEG markedly reduced the average APA IgM, with ~1.1, ~1.2 and ~0.7 μg/mL found in mice receiving 5 mg/kg 40 kDa PEG, 50 mg/kg 40 kDa PEG and 5 mg/kg 100 kDa PEG together with pegloticase, respectively, representing a ~7-, ~6- and ~10-fold lower APA IgM than mice receiving pegloticase administered with saline. We found no changes of mice body weight during the study (Supp Figure S3). Since 5 mg/kg of 40 kDa PEG most effectively suppressed pegloticase-mediated APA induction, we further evaluated whether it can also limit APA induction against larger doses of pegloticase, and observed similar extent of APA suppression (Supp Figure S4). Notably, our assay assesses APA that bind to the terminal PEG (e.g. methoxy-PEG) or the PEG backbone (anti-PEG antibody) concurrently, suggesting that the free PEG intervention could reduce immunogenicity and induction of both types of APA (Supp Figure S5). These results strongly underscore the effectiveness of adding free PEG to pegloticase in inhibiting pegloticase-induced APA.
Figure. 1. Induction of APA by pegloticase.
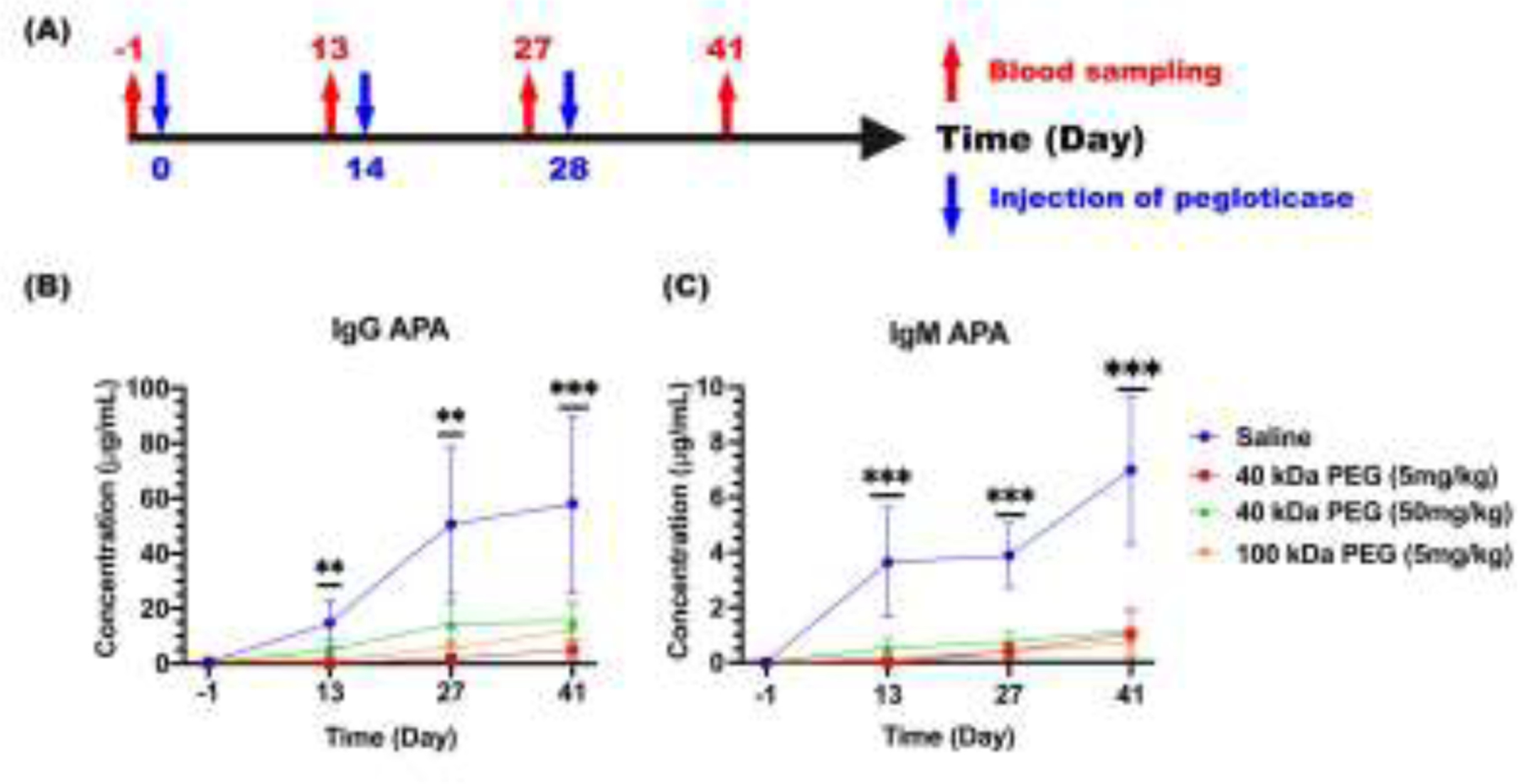
(A) Schematic illustration of the study design and sampling schedule evaluating APA titers induced by pegloticase given either with saline or different MW and amount of free PEG. Separate groups of mice were given these pegloticase solutions intravenously on Days 0, 14, and 28, and APA in blood was quantified 13 days after each injection as well as 1 day prior to the first injection on Days −1, 13, 27 and 41; (B) Anti-PEG IgG and (C) IgM concentrations in mice over time. Blue circle represents saline and pegloticase; red square represents 5 mg/kg 40 kDa free PEG and pegloticase; green triangle represents 50 mg/kg 40 kDa free PEG and pegloticase; and orange down-pointing triangle represents 5 mg/kg 100 kDa free PEG and pegloticase. Each specimen was measured in triplicate in two independent experiments, with the average and SEM reported (n=8). Statistical comparisons across groups were one-way ANOVA with Bonferroni-adjust for multiple comparisons, with *, **, and *** representing p < 0.05, 0.01, and 0.001, respectively.
3.2. Addition of free PEG to pegloticase maintains prolonged circulation of drug over multiple rounds of dosing as revealed by PET/CT imaging study
Since the efficacy of pegloticase therapy is directly related to the level of the drug in the circulation, we next evaluated the duration of circulation of pegloticase following multiple rounds of injection. The pegloticase pharmacokinetic (PK) profile can be readily measured by labeling pegloticase with 89Zirconium ([89Zr]), and quantifying the biodistribution in real time via positron emission tomography-computed tomography (PET/CT) imaging in live animals. For this study, we focused on determining the PK profile of pegloticase containing 5 and 50 mg/kg 40 kDa free PEG injected on day 42 (Groups 2 and 3). We compared these data against [89Zr]-pegloticase PK collected in mice that had received the same number of injections of pegloticase mixed with saline (Group 1), as well as naïve BALB/c mice (without any prior pegloticase-dosing, and thus no detectable APA) co-injected with saline and [89Zr]-pegloticase for the first time (Group 4) (Figure 2A). We performed dynamic scanning on a small number of animals to determine the biodistribution of pegloticase over the first hour post-injection, and collected static scans of PET and CT at select time points for all animals (1, 4, 24, 48, 72 and 96 hours). In Group 1 mice with APA induced by prior dosing of pegloticase with saline, the injection of [89Zr]-pegloticase on day 42 led to rapid label accumulation in liver within the first hour (Figure 2B). The signal in liver saturated by 24 hours post-injection, with negligible quantities detected in blood and other organs. In contrast, in APA-naïve mice receiving [89Zr]-pegloticase for the first time, as well as mice that had received multiple rounds of pegloticase containing 40 kDa free PEG prior to the dose of [89Zr]-pegloticase mixed with free PEG, we observed intense radioactive signal in the heart over the first 24 hours, indicating high concentration of circulating drugs in the blood. In these mice, the PET signal over the mouse body could be readily detected across the entire 96-hour duration of the study. In contrast to mice dosed with pegloticase diluted in saline, no liver accumulation was observed when free PEG was included with the pegloticase.
Figure. 2. PET/CT imaging over time in mice treated with [89Zr]-pegloticase with or without free PEG added.
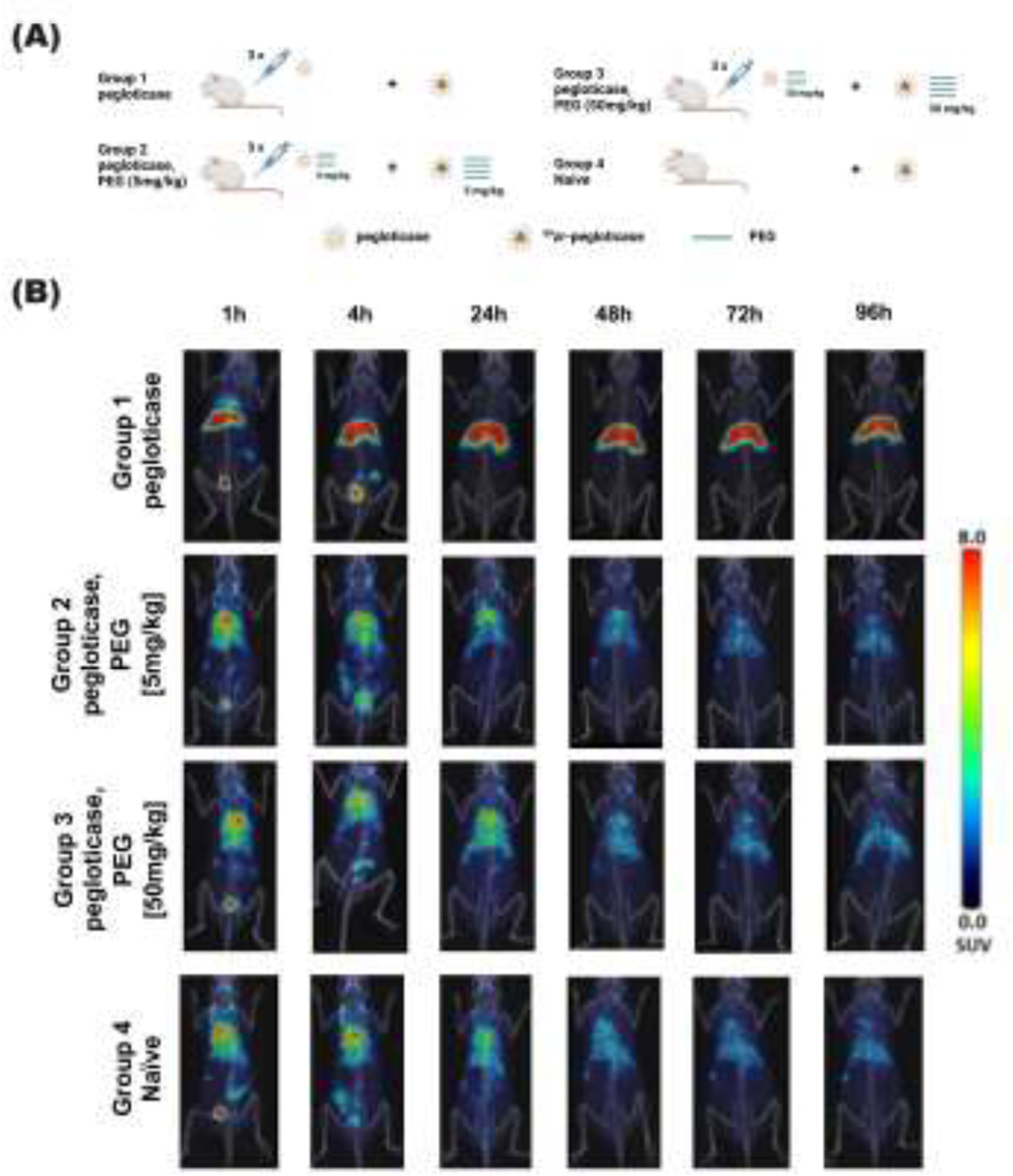
(A) Schematic figure of PET/CT study design. (B) PET/CT imaging was performed at different time points to assess pharmacokinetics and biodistribution of [89Zr]-pegloticase in four cohorts: mice previously dosed with 3 rounds of pegloticase every 2 weeks, comprised of pegloticase co-administered with saline (Group 1), 5 mg/kg free PEG (Group 2), 50 mg/kg free PEG (Group 3), as well as mice with no prior pegloticase dosing injected with saline and [89Zr]-pegloticase for the first time. For Groups 1 to 3, [89Zr]-pegloticase is dosed together with the corresponding saline or free PEG. Single PET coronal section was overlaid on corresponding CT MIP image to represent the biodistribution of [89Zr]-pegloticase at indicated time points.
To assess [89Zr]-pegloticase biodistribution over time, we focused on quantifying the PET signal over time in five regions of interests (ROI), including heart (representing plasma), liver, kidney, muscle and lung. We first analyzed the dynamic imaging data collected over the first hour. In mice with APA induced by repeated injections of pegloticase diluted in saline, the level of [89Zr]-pegloticase started to decrease in the blood and other organs and increase in the liver within the first five minutes post-injection, and the trend continued for the first hour (Group 1; Figure 3A). In contrast, in mice that had been receiving pegloticase containing either 5 mg/kg or 50 mg/kg free PEG (i.e., Groups 2 and 3), the concentration of [89Zr]-pegloticase in the heart was steady over the first hour, and markedly higher than signal in other organs (Figure 3B, 3C). The concentration of pegloticase in the liver of these mice was not appreciably different from that of kidney and lung, indicating the absence of liver accumulation. The biodistribution of [89Zr]-pegloticase in muscle was negligible in all groups.
Figure. 3. Dynamic PET/CT scanning of [89Zr]-pegloticase in mice receiving different interventions over the first 1 hour post infusion.
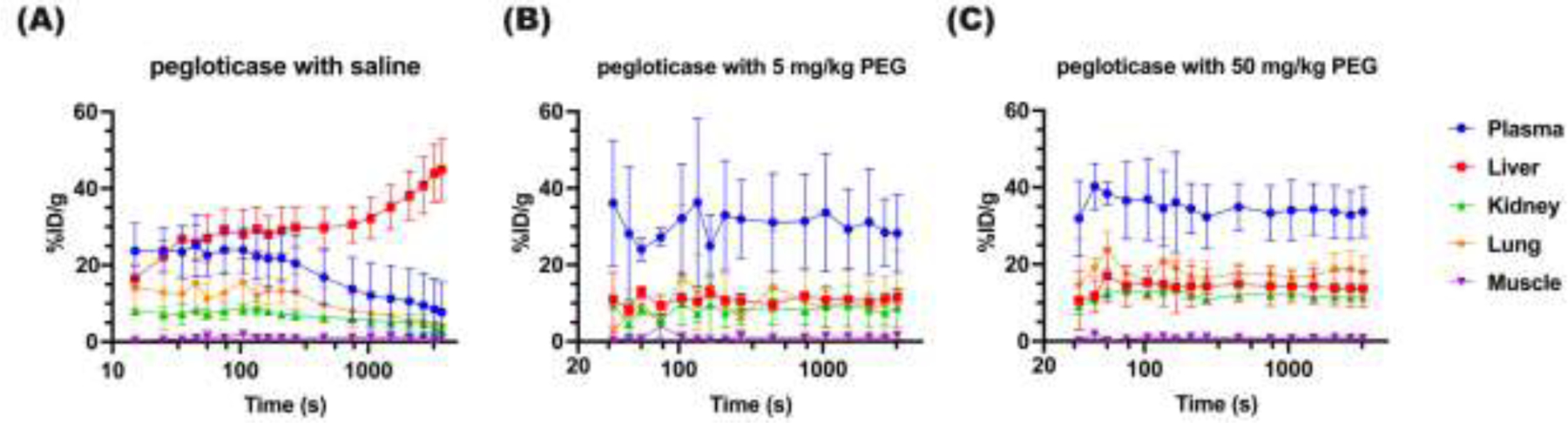
Mean 89Zr levels in various organs over the first 1 hour in mice treated with (A) [89Zr]-pegloticase with saline, (B) [89Zr]-pegloticase with 5 mg/kg free PEG, (C) [89Zr]-pegloticase with 50 mg/kg free PEG. Blue circle represents heart; red square represents liver; green triangle represents kidney; purple down-pointing triangle represents muscle; and orange diamond represents lung. Data represent mean ± SEM.
We next analyzed static scans acquired at 1, 4, 24, 48, 72, and 96 hours post-injection, and summarized the changes in mean percent injected dose per gram (%ID/g, adjusted for radioactivity decay) of the key ROIs in Figure 4 (signal not adjusted for the radioactivity decay of 89Zr was shown in Supp Figure S6). The amount of [89Zr]-pegloticase in the heart of mice with high titers of APA induced by repeated injection of pegloticase in saline (i.e. Group 1) was under 10% by the 1-hour time point, and under 3% by the 4-hour time point. Even at these very early time points, the levels of [89Zr]-pegloticase in the heart was ~16- to ~12-fold less than those of mice given either pegloticase in saline for the first time, or mice that have received multiple prior rounds of pegloticase containing free PEG. The level of [89Zr]-pegloticase in the heart further decreased over the first 24 hours to nearly non-detectable levels in mice repeatedly given pegloticase in saline. In contrast, in mice treated with pegloticase containing free PEG, the level of [89Zr]-pegloticase in the heart was only very modestly reduced (~12% and ~14% in mice treated with 5 mg/kg and 50 mg/kg PEG added to pegloticase prior to dosing) compared to naïve mice given pegloticase in saline for the first time on day 42. Importantly, the heart concentration only gradually decreased over time, with a half-life of roughly 71, 43 and 96 hours in mice receiving multiple rounds of pegloticase containing 5 mg/kg and 50 mg/kg of 40 kDa free PEG as well as naïve mice receiving pegloticase in saline for the first time, respectively (calculated from the 4hr timepoint onwards to reduce the alpha-phase distribution effect). The concentration of [89Zr]-pegloticase in heart of mice receiving pegloticase containing 5 mg/kg free PEG was not statistically different from those in naïve mice receiving pegloticase for the first time at all time points (Table 1). Sustained, high levels of [89Zr]-pegloticase accumulation in the liver over the full 96 hours was found in mice receiving repeated co-injections of pegloticase in saline only (Group 1), while no significant difference was found between Group 2 and Group 4 mice. Statistically difference was only found at the 96 hours post-injection time point between mice that had received multiple rounds of pegloticase containing 50 mg/kg PEG vs. naïve mice receiving pegloticase for the first time, in both heart and liver. Due to the extensive hepatic accumulation, the %ID/g of [89Zr]-pegloticase in kidney and lung were both less then 5% in Group 1 mice, compared with appreciably higher levels (5 – 25%) in Group 2 – 4 mice. Similar trend was found in spleens (Supp Figure S7). The concentration of [89Zr]-pegloticase in muscle was essentially at background levels among all groups of mice.
Figure. 4. Distribution of [89Zr]-pegloticase, with or without added free PEG, in different organs over time.
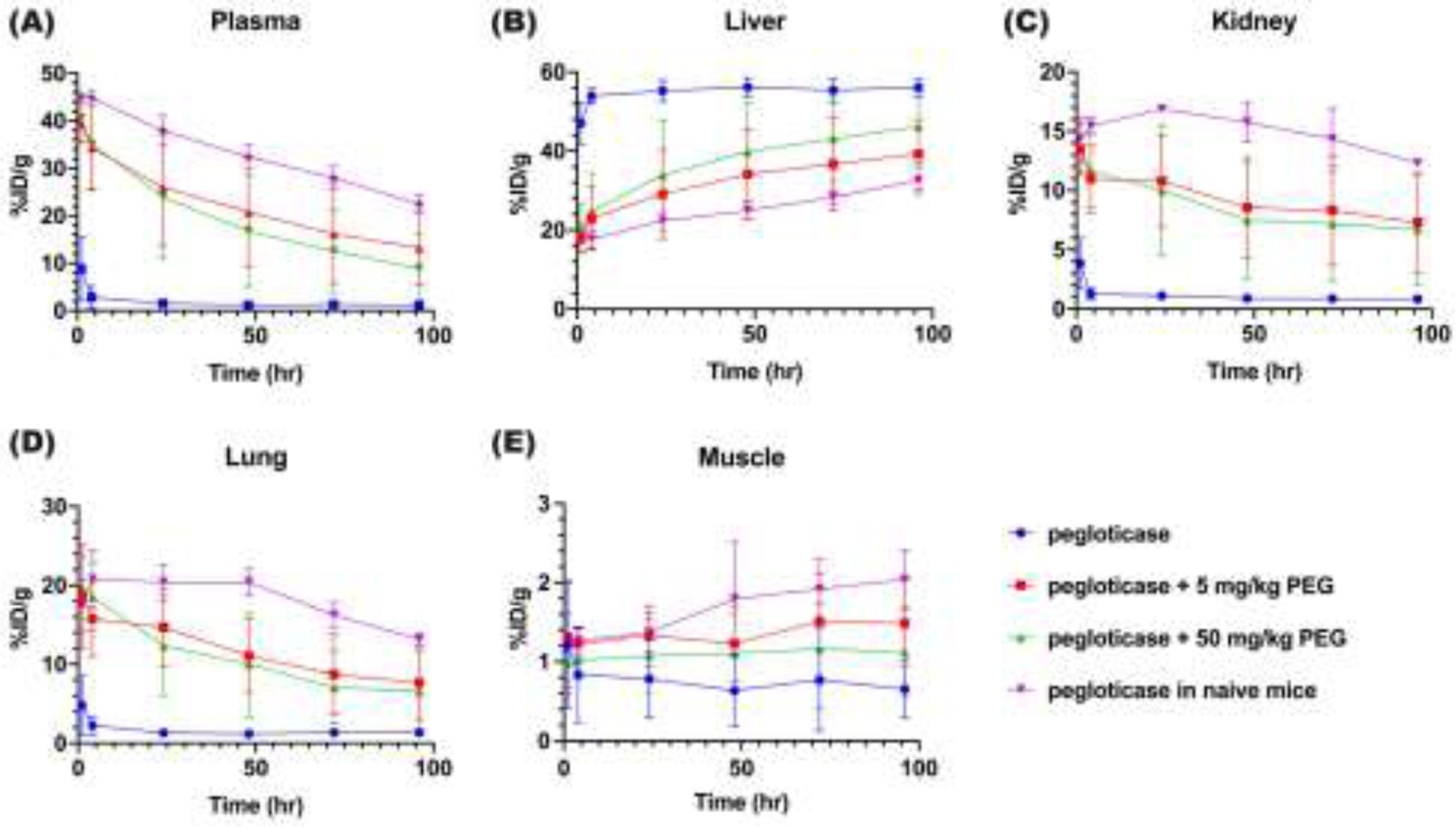
Mean [89Zr]-pegloticase concentration over the first 96 hours in (A) heart, (B) liver, (C) kidney, (D) lung and (E) muscle, as determined by PET/CT imaging and expressed as %ID per gram of tissue. Blue circle represents mice infused with [89Zr]-pegloticase and saline (Group 1); Red square represents mice infused with [89Zr]-pegloticase containing 5mg/kg free PEG (Group 2); Green triangle represents [89Zr]-pegloticase containing 50mg/kg free PEG (Group 3); purple down-pointing triangle represents APA-naïve mice co-infused with [89Zr]-pegloticase and saline for the first time (Group 4). The signal was adjusted for radioactive decay. Data represent mean ± SEM; please refer to Table 1 for statistical comparisons.
Table 1.
Statistical analysis of difference of %ID/g quantified by PET imaging of liver and heart (proxy for blood) between treatments
| Saline vs. 5mg/kg PEG | Saline vs. 50mg/kg PEG | Saline vs. Naïve | 5mg/kg PEG vs. 50mg/kg PEG | 5mg/kg PEG vs. Naïve | 50mg/kg PEG vs. Naïve | |
|---|---|---|---|---|---|---|
| Heart 4h | p<0.001*** | p<0.001*** | p<0.001*** | p>0.05 | p>0.05 | p>0.05 |
| Heart 24h | p<0.001*** | p<0.001*** | p<0.001*** | p>0.05 | p>0.05 | p>0.05 |
| Heart 48h | p<0.001*** | p<0.01** | p<0.001*** | p>0.05 | p>0.05 | p>0.05 |
| Heart 96h | p<0.01** | p<0.05* | p<0.001*** | p>0.05 | p>0.05 | p<0.05* |
| Liver 4h | p<0.001*** | p<0.001*** | p<0.001*** | p>0.05 | p>0.05 | p>0.05 |
| Liver 24h | p<0.001*** | p<0.01** | p<0.001*** | p>0.05 | p>0.05 | p>0.05 |
| Liver 48h | p<0.001*** | p<0.01** | p<0.001*** | p>0.05 | p>0.05 | p>0.05 |
| Liver 96h | p<0.001*** | p<0.05* | p<0.001*** | p>0.05 | p>0.05 | p<0.05* |
Student’s t-test was applied to determine the difference among group, and P values were summarized in the table.
denotes p < 0.05;
denotes p < 0.01;
denotes p < 0.001.
To assess how differences in the measured pharmacokinetics may translate to potential activity, we calculated the area under curve (AUC) in heart and different organs from the PET imaging over the first four days for each group. Relative to [89Zr]-pegloticase diluted in saline given for the first time to APA-naïve mice, the AUC0–96h of [89Zr]-pegloticase in the heart was reduced ~95%, ~34% and ~43% in mice repeatedly dosed with pegloticase and saline (Group 1), pegloticase containing 5 mg/kg PEG (Group 2) or pegloticase containing 50 mg/kg PEG (Group 3), respectively (Figure 5A). Not surprisingly, [89Zr]-pegloticase AUC0–96h in the liver of mice repeatedly receiving pegloticase in saline only (Group 1) was markedly higher than those of other groups (Figure 5B). Corresponding, AUC0–96h of kidney and lung in Group 1 mice was remarkedly lower than those in Groups 2–4 mice. AUC in the muscle is negligible for all groups given limited muscle distribution of [89Zr]-pegloticase.
Figure. 5. AUC of the biodistribution profiles of [89Zr]-pegloticase, with or without added free PEG.
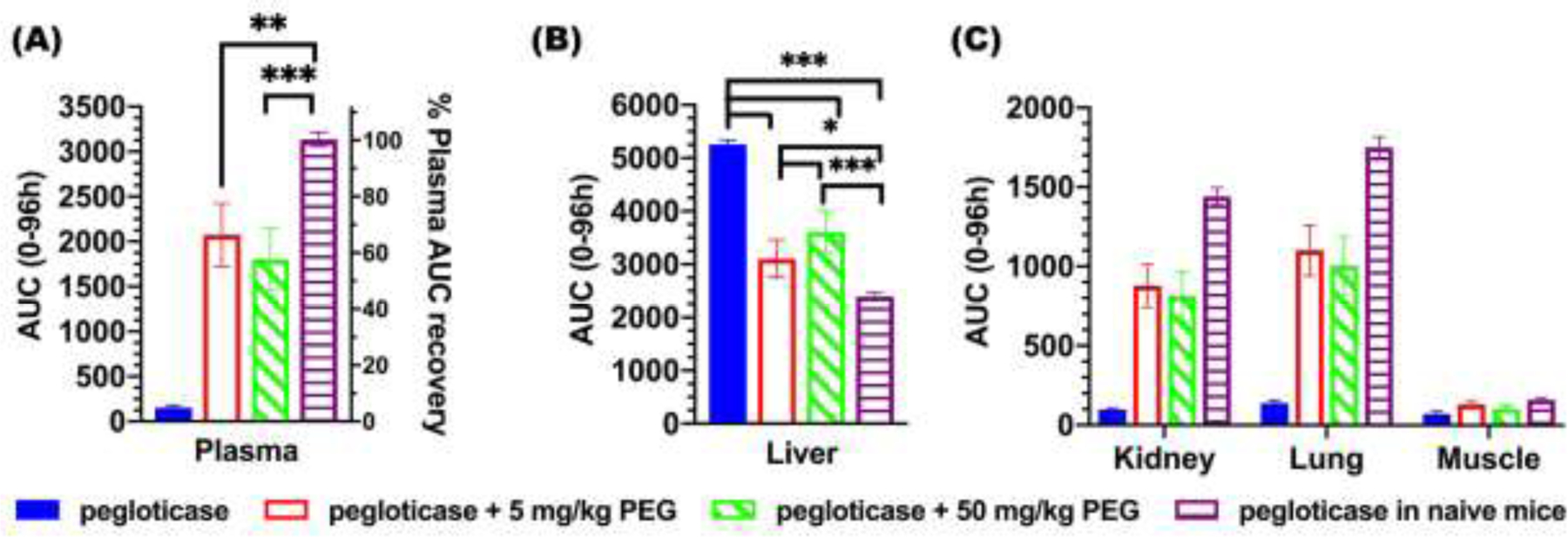
AUC for concentration of [89Zr]-pegloticase over time in different organs: (A) heart, (B) liver, and (C) kidney, lung and muscle. Data depict mean ± bars represent standard deviation. Statistical comparisons across groups were one-way ANOVA with Bonferroni-adjust for multiple comparisons, with *, **, and *** representing p < 0.05, 0.01, and 0.001, respectively.
3.3. Correlating heart and liver levels of [89Zr]-pegloticase to serum APA levels.
To further confirm the relationship between APA and [89Zr]-pegloticase distribution, we compared the measured [89Zr]-pegloticase levels in the heart and the liver with levels of APA IgG measured before the PET/CT study (Figure 6A). In good agreement with our expectation, mice with higher serum APA IgG (≥ 30 μg/mL) tend to possess lower levels of [89Zr]-pegloticase in the heart (i.e. reflecting levels in the blood) and higher levels in the liver (key organ of distribution, see Figure 2), particularly at the shorter time points. At all time points, we found a correlation coefficient of ~ −0.73 between APA IgG concentrations and plasma levels of [89Zr]-pegloticase (p < 0.01), as well as a correlation coefficient of over 0.66 regarding liver levels of [89Zr]-pegloticase (p < 0.01). We also saw a similar relationship between serum APA IgM level and [89Zr]-pegloticase levels in the heart and liver (Supp Figure S8). These results are consistent with serum APA as a key mediator of rapid clearance of pegloticase from the blood into the liver.
Figure. 6. Concentration of [89Zr]-pegloticase in blood (heart) and liver over time in mice with different levels of IgG APA.
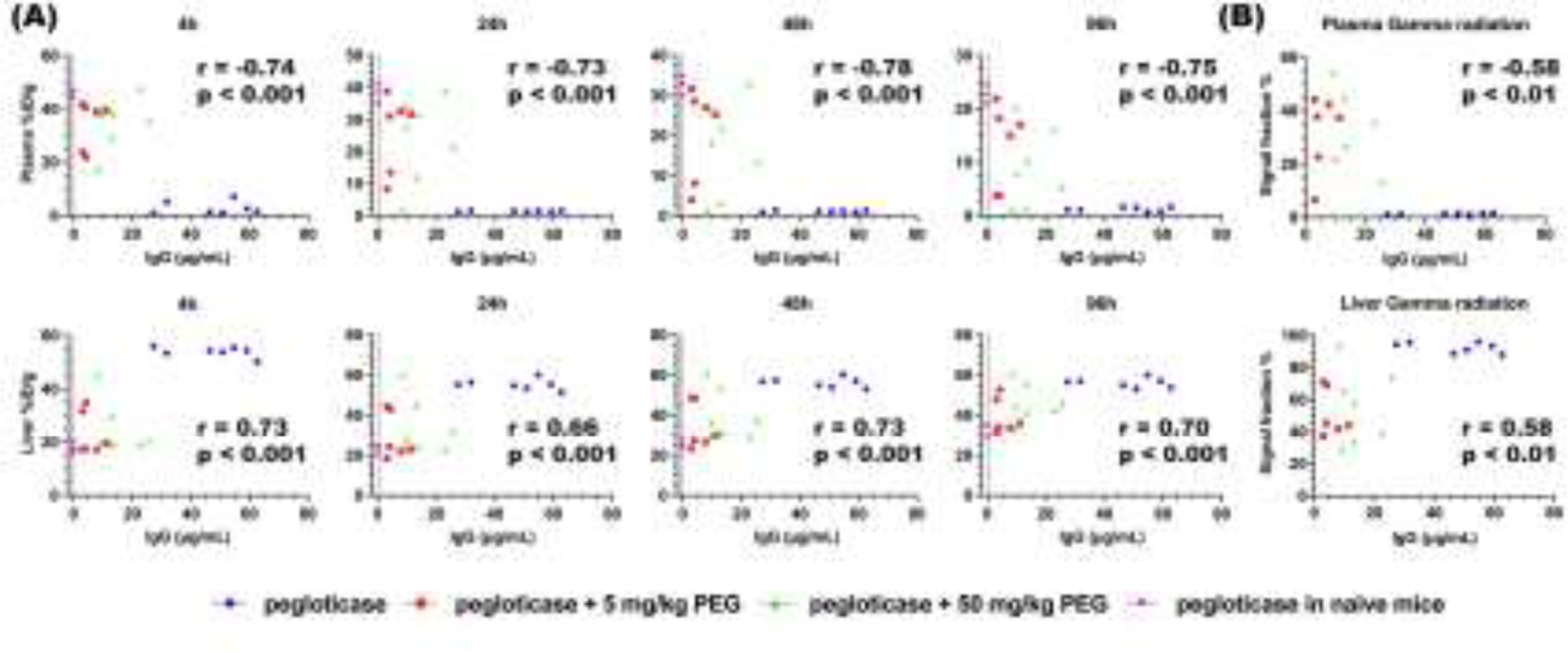
(A) The concentration of [89Zr]-pegloticase quantified by PET/CT imaging at 4h, 24h, 48h and 96h, expressed as %ID per gram of tissue, as a function of IgG APA in (A) blood and liver. (B) Signal fraction of blood and liver from gamma counter as a function of IgG APA. Blue circle represents mice infused with pegloticase only (i.e. formulated with saline); red square represents mice infused with pegloticase containing 5mg/kg free PEG; green triangle represents pegloticase containing 50mg/kg free PEG; pink inverted triangle represents naïve mice receiving pegloticase only. Correlation was analyzed by nonparametric Spearman correlation, with correlation coefficient r reported, as well as p value of each correlation assay.
We harvested these organs after the PET/CT study and assessed gamma radiation via gamma counter. As expected, results from gamma counter were in strong agreement with the results from PET/CT scans (Supp Figure S9). We also found similar correlation between gamma counter-derived concentrations of [89Zr]-pegloticase in the blood and liver vs. the serum concentration of APA, with greater elimination from the heart to the liver in mice with higher APA titers (Figure 6B). We saw a correlation coefficient of ~ −0.58 between APA IgG concentrations and gamma counting signal in plasma (p < 0.01), and 0.58 between IgG concentrations and gamma counting signal in liver (p < 0.01).
3.4. Free PEG blocked PEGylated protein from binding with APA BCR+ cells.
To begin to understand how free PEG may inhibit APA production, we generated cells expressing B cell receptors (BCRs) that can specifically bind PEG, and assessed whether free PEG can effectively inhibit interaction between the cells and a model PEGylated protein drug (PEG-BSA). Consistent with our expectation, the inclusion of free PEG largely abrogated the binding between PEG-BSA and anti-PEG BCR+ cells to level comparable to controls cells without anti-PEG BCR (i.e. anti-PEG BCR-) (Figure 7).
Figure. 7. Free PEG blocks interactions between anti-PEG BCR and PEG-BSA.
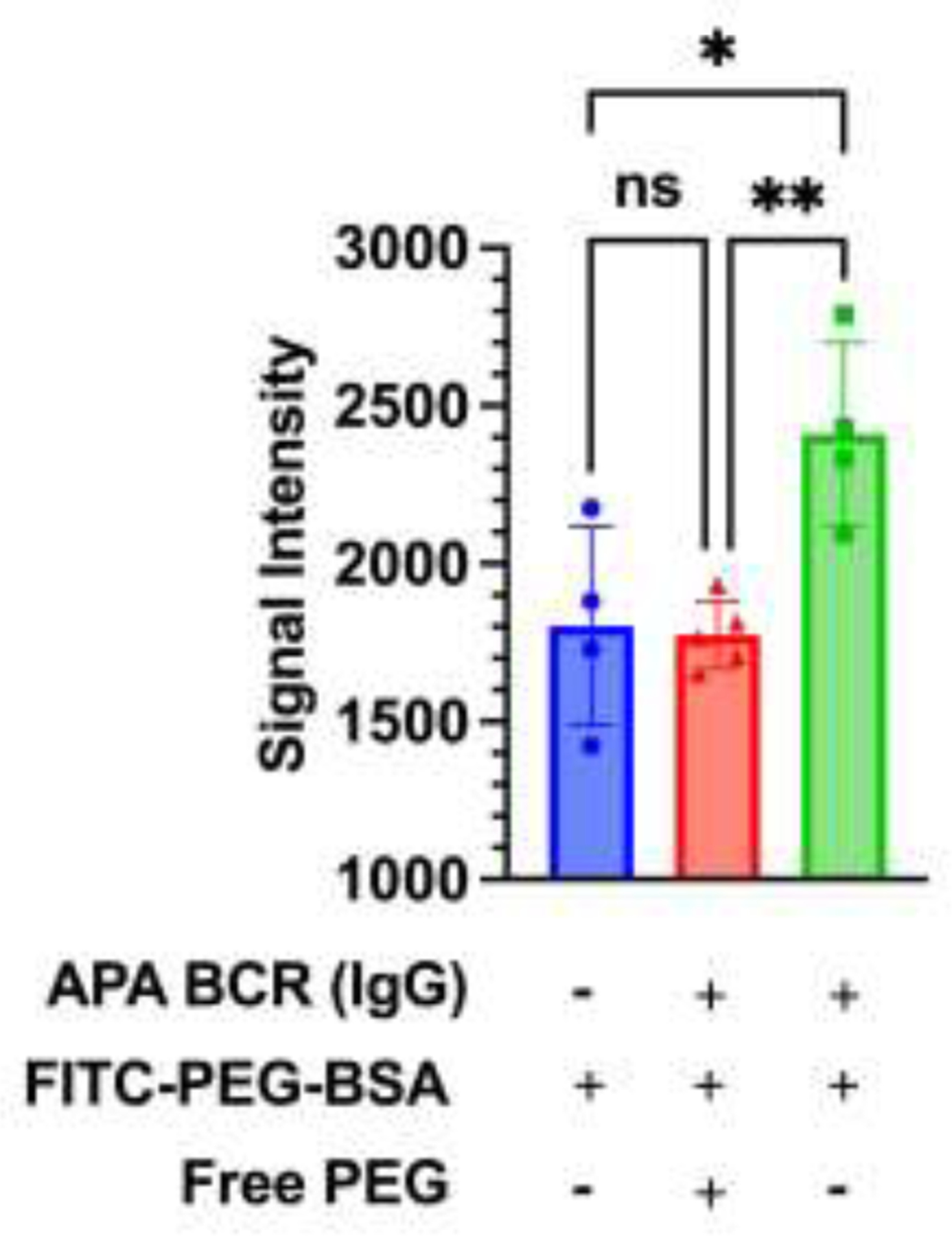
Relative fluorescence intensity from FITC-PEG-BSA. Blue circle represents unmodified Expi293 cells incubated with PEG-BSA (negative control); red triangle represents cells possessing anti-PEG BCRs incubated with PEG-BSA and free PEG; green square represents the same cells incubated with PEG-BSA only. Statistical comparisons across groups were one-way ANOVA with Bonferroni-adjust for multiple comparisons, with *, **, and *** representing p < 0.05, 0.01, and 0.001, respectively.
4. Discussion
Over the past 20 years, PEG has been perhaps the most used polymer in the formulation of various drugs [36, 37]. In addition to protein drugs, PEG is also frequently incorporated in various nanoparticles systems, including the recent mRNA COVID vaccines from Pfizer and Moderna that have been dosed to more than a billion people worldwide [38, 39]. We believe the increasing adoption of PEGylated drugs underscores both the safety of PEG, and also the effectiveness of PEGylation as a strategy to enhance circulation and efficacy. We thus believe PEGylation will continue to be a mainstay in many therapeutics in the foreseeable future, particularly given the increasing number of biologics in clinical development.
Despite the overall success of PEGylation, allergic reactions to PEG have been noted for several PEGylated drugs, including PEG-asparaginase, PEG-interferon alpha, PEGylated G-CSF (pegfilgrastim) and two COVID mRNA vaccines formulated with lipid-PEG conjugates [40–44], with typical adverse events ranging from infusion reactions to anaphylaxis [45–47]. Reported hypersensitivity was often correlated with the presence of IgG and IgE APA [48], and correlation between IgM APA and infusion reactions was also demonstrated in a porcine model [49]. Although the precise mechanism of adverse reactions to COVID mRNA vaccines in patients with history of allergy remains unclear, some researchers have also hypothesized the involvement of APA, due to the inclusion of PEG to stabilize mRNA, and the boost of APA in some vaccinated patients [50–53]. APA-induced allergic responses, and APA-mediated ABC that compromises the PK, can directly impede the safe and efficacious use of select PEGylated drugs. Unfortunately, there is currently no readily employable strategy to manage APA, other than to simply discontinue and switch treatments, or to employ broad immunosuppression. While there is active interest to develop less immunostimulatory PEGylated therapeutics [15, 54], there is generally no method to tune the immunogenicity of existing PEGylated drugs with molecular specificity. These realities motivated our group to explore whether high MW free PEG can provide a safe, efficacious, and easily translatable solution to enable the use of PEGylated drugs in patients at risk of developing substantial APA titers. Our efforts culminated in the current report, which demonstrates, for the first time, that co-administration of PEG can both tune the immunogenicity and effectively limit APA induction, and prolong the PK and apparent circulation of a clinically relevant PEGylated protein drug over the course of multiple rounds of injection in mice. We believe the simplicity of the free PEG approach, coupled with the effectiveness seen here, makes our strategy highly translatable in a clinical setting.
We showed here that the presence of free PEG can effectively attenuate the immunogenicity of pegloticase (Figure 1), including reducing both IgG and IgM APA following multiple rounds of injection. While the precise mechanism remains to be further investigated, the principle likely involves substituting the more immunogenic PEGylated drugs with less immunogenic free PEG. We showed that free PEG can indeed compete with PEGylated proteins and blocked their binding to cells with surface anti-PEG BCRs, consistent with free PEG binding to anti-PEG BCR (Figure 7). We speculate the difference in immunogenicity is a direct consequence of how their physical form interfaces with BCRs. When multiple PEG molecules are conjugated to a protein or nanoparticle, their effective footprint is much larger (e.g. pegloticase is ~540 kDa, and PEG-liposomes are ~100 nm in diameter), allowing each PEGylated drug entity to more effectively crosslink BCRs on the surface of APA+ B cells, which in turn stimulates the secretion of APA. In contrast, while free PEG polymers (~40 kDa) can similarly bind BCR, their smaller footprint coupled with their highly flexible nature makes them much less efficient at crosslinking multiple BCRs, particularly when PEG is dosed at molar excess. Compared to pegloticase, free PEG is unlikely to be digested by antigen presenting cells (APC) and presented in major histocompatibility complex (MHC), thus potentially blocking T-dependent B cell activation. Consistent with this notion, PEG on its own is poorly immunogenic, as are many smaller PEGylated proteins [6, 55, 56]. In addition, free PEG likely also competitively inhibits the formation of large immune complexes comprising APA with PEGylated drugs, thus further reducing immunostimulation. While shorter MW PEG may also similarly competitively inhibit interactions between PEGylated drugs with the immune system, their rapid renal clearance places severe limits to their effectiveness over time. Our use of high MW free PEG allows us to achieve both effective reduction of PEG-immunogenicity, and maintain the effect over time. Meanwhile, toxicity is rarely detected with repeated doses of free PEG across various small and large animal studies [55, 57–59], including high MW free PEG tested at doses up to 200 mg/kg/week. Thus, we expect our strategy to be both safe and effective in restoring PEGylated drugs that require frequent dosing.
The use of free PEG to competitively inhibit APA binding and reduce APA-induction contrasts sharply with current efforts focusing on broad immunosuppression using immunomodulators [60, 61]. For pegloticase, the most advance immunosuppression-based intervention utilizes MTX dosed weekly one month prior to the first dosing of pegloticase, with MTX dosing continuing until the end of the study (ClinicalTrials.gov: NCT03635957) [20]. During the six-month of pegloticase treatment, complete functions were detected in 11/14 patients, and all patients tolerated the MTX- pegloticase regimen. Given these promising results, several similar clinical trials are ongoing to determine the efficacy of co-administration of MTX in various conditions including different doses of pegloticase, different infusion speed, and in patients who previously failed with pegloticase treatment. In particular a large (n=152) randomized controlled trial of co-treated uncontrolled gout patients with pegloticase with either methotrexate 15 mg or placebo coadministration resulted in a higher response rate with the addition of methotrexate (71% vs 38.5% response rates). These results supported the recent FDA approval for expanded labeling for Krystexxa® to include concomitant use with methotrexate.
While the clinical trial results achieved using MTX are exceptionally encouraging, some patients may benefit from alternative interventions such as free PEG explored here. This includes patients who continue to experience elevated levels of uric acid despite the concomitant use of MTX; since pegloticase is a last line therapy, no alternatives currently exist for patients treated with pegloticase who become non-responders. This may also include patients who experience side effects or have other underlying medical conditions that may preclude the use of MTX. It remains to be determined whether the concomitant use of MTX requires a month-long lead-in MTX dosing as is typically done based on currently available data. Given that gout is a highly debilitating inflammatory condition, some patients may benefit from interventions that are non-immunosuppressant, such as PEG, and that may therefore avoid a lead-in dosing with immunomodulators such as MTX. Since free PEG can immediately limit APA-mediated pegloticase clearance and reduce induction of APA, we speculate that no lead-in dosing period will be required for PEG. Coupled with the low costs (USP-grade PEG is readily available at scale (<$1/g)) and well-established track record of safety of PEG in humans, we believe the free PEG intervention strategy should be explored further in preclinical and clinical studies.
It’s useful to compare the effectiveness of PK restoration observed here to our earlier studies, both of which focused on pre-infusion of high dose of free PEG to animals with high titers of APA already present. In our 2019 study testing pre-infusion of 550 mg/kg 40 kDa free PEG 30 mins before administration of Doxil, a PEGylated liposome, we were able to recover ~60% of the AUC of Doxil when given to APA naïve mice, whereas APA+ mice given Doxil without PEG infusion recovered only ~5% compared to APA naïve mice [55]. More recently, we showed that pre-infusion of 550 mg/kg 40 kDa free PEG into mice with high titers of pegloticase-induced APA 30 mins before the pegloticase dosing effectively restored the PK, with ~80% recovery of AUC compared to that of pegloticase given for the first time to naïve mice [25]. These data support and extend the present findings where, co-administration of free PEG with pegloticase significantly reduced APA induction, and led to a similar extent of AUC recovery (~66%) compared to pegloticase given to APA-naïve animal, despite the use of >100-fold less PEG. We found no significant difference in IgG and IgM APA between the three formulations (5 mg/kg 40 kDa PEG, 50 mg/kg 40 kDa PEG, 5 mg/kg 100 kDa PEG) tested, though 5 mg/kg 40 kDa PEG showed slightly more effective inhibition than either higher MW PEG (100 kDa) or higher dose PEG (50 mg/kg). Both dose levels of 40 kDa PEG significantly prolonged pegloticase circulation and reduced hepatic clearance, with 5 mg/kg dose exhibiting marginally better (but not statistically significant) effectiveness. We conclude that the 5 mg/kg dose evaluated here is sufficient to reduce induction of IgG and IgM APA against pegloticase to a level where any induced APA can be sufficiently competitively inhibited from binding and clearing a large fraction of pegloticase. Our preference is to utilize the lowest effective dose of any intervention; thus, we prefer 5 mg/kg over 50 mg/kg dose for further clinical development. This reduction of PEG dose will also help decrease concerns about the safety of the free PEG intervention, and makes free PEG intervention a clinical translatable strategy. Due to the greater track record of safety of 40 kDa PEG over 100 kDa PEG, we also prefer using 40 kDa PEG. Comparing with our earlier work showing APA reduction by pre-infusion of free PEG, we showed that free PEG could be dosed along with the dosage regimen of pegloticase, which significantly increases patient adherence. In this study, we showed for the first time that we can effectively reduce the immunogenicity of a clinically relevant drug, potentially paving the way of utilizing the same approach to tune the immunogenicity of other polymer-modified therapeutics.
Supplementary Material
Statement of significance.
A major challenge with engineering materials for drug delivery is their interactions with the immune system. For instance, our body can produce high levels of anti-PEG antibodies (APA). Unfortunately, the field currently lack tools to limit immunostimulation or overcome pre-existing anti-PEG antibodies, without using broad immunosuppression. Here, we showed that simply introducing free PEG into a clinical formulation of PEG-uricase can effectively limit induction of anti-PEG antibodies, and restore their prolonged circulation upon repeated dosing. Our work offers a readily translatable method to safely and effectively restore the use PEG-drugs in patients with PEG-immunity, and provides a template to use unconjugated polymers with low immunogenicity to regulate interactions with the immune system for other polymer-modified therapeutics.
Acknowledgments.
We like to thank the staff at the UNC Animal Studies Core. The Small animal imaging core facility is partially supported by an NIH grant P30-CA016086. The PET/CT imaging equipment was obtained through NIH high-end instrumentation grant, S10-OD023611-01. Schematic figures were created with BioRender.com.
Funding.
This work was supported by The David and Lucile Packard Foundation (2013–39274, S.K.L), National Institutes of Health (T32-HL069768; AMT, R01 HL141934; S.K.L.), P.E.O. International (A.M.T), and Eshelman Institute of Innovation (S.K.L). The BRIC Small Animal Imaging (S.A.I) core facility and UNC Lineberger Animal Core are supported in part by an NCI Grant P30-CA016086. The PET/CT equipment is supported by the NIH shared instrumentation grant (1S10OD023611, ZL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest. S.K.L. is an inventor of patents on the use of free PEG to block APA. The terms of these arrangements are managed by UNC-CH in accordance with its conflict-of-interest policies. A.C.N., M.S.B. and B.L. are employees of, and have stock in, Horizon Therapeutics.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
S.K.L. is an inventor of patents on the use of free PEG to block APA. The terms of these arrangements are managed by UNC-CH in accordance with its conflict-of-interest policies. A.C.N., M.S.B. and B.L. are employees of, and have stock in, Horizon Therapeutics.
References:
- 1.Chen BM, Cheng TL, and Roffler SR, Polyethylene Glycol Immunogenicity: Theoretical, Clinical, and Practical Aspects of Anti-Polyethylene Glycol Antibodies. ACS Nano, 2021. 15(9): p. 14022–14048. [DOI] [PubMed] [Google Scholar]
- 2.Klibanov AL, Maruyama K, Torchilin VP, and Huang L, Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett, 1990. 268(1): p. 235–7. [DOI] [PubMed] [Google Scholar]
- 3.Veronese FM and Pasut G, PEGylation, successful approach to drug delivery. Drug Discov Today, 2005. 10(21): p. 1451–8. [DOI] [PubMed] [Google Scholar]
- 4.Ju Y, Kelly HG, Dagley LF, Reynaldi A, Schlub TE, Spall SK, Bell CA, Cui J, Mitchell AJ, Lin Z, Wheatley AK, Thurecht KJ, Davenport MP, Webb AI, Caruso F, and Kent SJ, Person-Specific Biomolecular Coronas Modulate Nanoparticle Interactions with Immune Cells in Human Blood. ACS Nano, 2020. 14(11): p. 15723–15737. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q and Lai SK, Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2015. 7(5): p. 655–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter AW and Akerblom E, Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int Arch Allergy Appl Immunol, 1983. 70(2): p. 124–31. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu T, Ichihara M, Yoshioka Y, Ishida T, Nakagawa S, and Kiwada H, Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biol Pharm Bull, 2012. 35(8): p. 1336–42. [DOI] [PubMed] [Google Scholar]
- 8.Lipsky PE, Calabrese LH, Kavanaugh A, Sundy JS, Wright D, Wolfson M, and Becker MA, Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res Ther, 2014. 16(2): p. R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Q, Jacobs TM, McCallen JD, Moore DT, Huckaby JT, Edelstein JN, and Lai SK, Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal Chem, 2016. 88(23): p. 11804–11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalil A, Wurthwein G, Golitsch J, Hempel G, Fobker M, Gerss J, Moricke A, Zimmermann M, Smisek P, Zucchetti M, Nath C, Attarbaschi A, Von Stackelberg A, Gokbuget N, Rizzari C, Conter V, Schrappe M, Boos J, and Lanvers-Kaminsky C, Pre-existing antibodies against polyethylene glycol reduce asparaginase activities on first administration of pegylated E. coli asparaginase in children with acute lymphocytic leukemia. Haematologica, 2022. 107(1): p. 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, and Garratty G, Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer, 2007. 110(1): p. 103–11. [DOI] [PubMed] [Google Scholar]
- 12.Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, Rusconi CP, and Hershfield MS, Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol, 2016. 137(5): p. 1610–1613 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, Vazquez-Mellado J, White WB, Lipsky PE, Horowitz Z, Huang W, Maroli AN, Waltrip RW 2nd, Hamburger SA, and Becker MA, Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA, 2011. 306(7): p. 711–20. [DOI] [PubMed] [Google Scholar]
- 14.Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, and Sundy JS, Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther, 2014. 16(2): p. R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoang Thi TT, Pilkington EH, Nguyen DH, Lee JS, Park KD, and Truong NP, The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers (Basel), 2020. 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Witteloostuijn SB, Pedersen SL, and Jensen KJ, Half-Life Extension of Biopharmaceuticals using Chemical Methods: Alternatives to PEGylation. ChemMedChem, 2016. 11(22): p. 2474–2495. [DOI] [PubMed] [Google Scholar]
- 17.Qi Y and Chilkoti A, Protein-polymer conjugation-moving beyond PEGylation. Curr Opin Chem Biol, 2015. 28: p. 181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y, Yuan Z, Zhang P, and Jiang S, Zwitterlation mitigates protein bioactivity loss in vitro over PEGylation. Chem Sci, 2018. 9(45): p. 8561–8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P, Jain P, Tsao C, Yuan Z, Li W, Li B, Wu K, Hung HC, Lin X, and Jiang S, Polypeptides with High Zwitterion Density for Safe and Effective Therapeutics. Angew Chem Int Ed Engl, 2018. 57(26): p. 7743–7747. [DOI] [PubMed] [Google Scholar]
- 20.Botson JK, Tesser JRP, Bennett R, Kenney HM, Peloso PM, Obermeyer K, LaMoreaux B, Weinblatt ME, and Peterson J, Pegloticase in Combination With Methotrexate in Patients With Uncontrolled Gout: A Multicenter, Open-label Study (MIRROR). J Rheumatol, 2021. 48(5): p. 767–774. [DOI] [PubMed] [Google Scholar]
- 21.Freyne B, A Case Report of Immunosuppressant MedicationAssociated Polyarticular Tophaceous Gout Successfully Treated Using the Polyethylene GlycolConjugated Uricase Enzyme Pegloticase. Transplant Proc, 2018. 50(10): p. 4099–4101. [DOI] [PubMed] [Google Scholar]
- 22.Khanna PP, Khanna D, Cutter G, Foster J, Melnick J, Jaafar S, Biggers S, Rahman A, Kuo HC, Feese M, Kivitz A, King C, Shergy W, Kent J, Peloso PM, Danila MI, and Saag KG, Reducing Immunogenicity of Pegloticase With Concomitant Use of Mycophenolate Mofetil in Patients With Refractory Gout: A Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol, 2021. 73(8): p. 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botson JK and Peterson J, Pretreatment and Coadministration With Methotrexate Improved Durability of Pegloticase Response: An Observational, Proof-of-Concept Case Series. J Clin Rheumatol, 2022. 28(1): p. e129–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HORIZON. FDA Approves KRYSTEXXA® (pegloticase) Injection Co-Administered With Methotrexate. 2022. [cited 2022 July 8]; Available from: https://ir.horizontherapeutics.com/news-releases/news-release-details/fda-approves-krystexxar-pegloticase-injection-co-administered.
- 25.Talkington AM, McSweeney MD, Zhang T, Li Z, Nyborg AC, LaMoreaux B, Livingston EW, Frank JE, Yuan H, and Lai SK, High MW polyethylene glycol prolongs circulation of pegloticase in mice with anti-PEG antibodies. J Control Release, 2021. 338: p. 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vosjan MJ, Perk LR, Visser GW, Budde M, Jurek P, Kiefer GE, and van Dongen GA, Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protoc, 2010. 5(4): p. 739–43. [DOI] [PubMed] [Google Scholar]
- 27.Davies B and Morris T, Physiological parameters in laboratory animals and humans. Pharm Res, 1993. 10(7): p. 1093–5. [DOI] [PubMed] [Google Scholar]
- 28.Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, and Beliles RP, Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health, 1997. 13(4): p. 407–84. [DOI] [PubMed] [Google Scholar]
- 29.Kaliss N and Pressman D, Plasma and blood volumes of mouse organs, as determined with radioactive iodoproteins. Proc Soc Exp Biol Med, 1950. 75(1): p. 16–20. [DOI] [PubMed] [Google Scholar]
- 30.Pesch T, Bonati L, Kelton W, Parola C, Ehling RA, Csepregi L, Kitamura D, and Reddy ST, Molecular Design, Optimization, and Genomic Integration of Chimeric B Cell Receptors in Murine B Cells. Front Immunol, 2019. 10: p. 2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepard BD, Natarajan N, Protzko RJ, Acres OW, and Pluznick JL, A cleavable N-terminal signal peptide promotes widespread olfactory receptor surface expression in HEK293T cells. PLoS One, 2013. 8(7): p. e68758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X, Williams JZ, Chang R, Li Z, Burnett CE, Hernandez-Lopez R, Setiady I, Gai E, Patterson DM, Yu W, Roybal KT, Lim WA, and Desai TA, DNA scaffolds enable efficient and tunable functionalization of biomaterials for immune cell modulation. Nature Nanotechnology, 2021. 16(2): p. 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, Sun F, Liu S, and Jiang S, Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J Control Release, 2016. 244(Pt B): p. 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganson NJ, Kelly SJ, Scarlett E, Sundy JS, and Hershfield MS, Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther, 2006. 8(1): p. R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundy JS, Ganson NJ, Kelly SJ, Scarlett EL, Rehrig CD, Huang W, and Hershfield MS, Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum, 2007. 56(3): p. 1021–8. [DOI] [PubMed] [Google Scholar]
- 36.Knop K, Hoogenboom R, Fischer D, and Schubert US, Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl, 2010. 49(36): p. 6288–308. [DOI] [PubMed] [Google Scholar]
- 37.Suk JS, Xu Q, Kim N, Hanes J, and Ensign LM, PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev, 2016. 99(Pt A): p. 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, and Weissman D, Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines (Basel), 2021. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Eygeris Y, Gupta M, and Sahay G, Self-assembled mRNA vaccines. Adv Drug Deliv Rev, 2021. 170: p. 83–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakatani A, Doi Y, Matsuda T, Sasai Y, Nishida N, Sakamoto M, Uenoyama N, Matsumoto Y, and Kinoshita K, Protracted anaphylaxis developed after peginterferon alpha-2a administration for chronic hepatitis C. World J Gastroenterol, 2015. 21(9): p. 2826–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbott LS, Zakova M, Shaikh F, Shewaramani N, Punnett A, and Dupuis LL, Allergic Reactions Associated with Intravenous Versus Intramuscular Pegaspargase: A Retrospective Chart Review. Paediatr Drugs, 2015. 17(4): p. 315–21. [DOI] [PubMed] [Google Scholar]
- 42.McSweeney MD, Mohan M, Commins SP, and Lai SK, Anaphylaxis to Pfizer/BioNTech mRNA COVID-19 Vaccine in a Patient With Clinically Confirmed PEG Allergy. Front Allergy, 2021. 2: p. 715844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mark C, Gupta S, Punnett A, Upton J, Orkin J, Atkinson A, Clarke L, Heisey A, McGovern C, and Alexander S, Safety of administration of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young adults with a history of acute lymphoblastic leukemia and allergy to PEG-asparaginase. Pediatr Blood Cancer, 2021. 68(11): p. e29295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dadla A, Tannenbaum S, Yates B, and Holle L, Delayed hypersensitivity reaction related to the use of pegfilgrastim. J Oncol Pharm Pract, 2015. 21(6): p. 474–7. [DOI] [PubMed] [Google Scholar]
- 45.Banerji A, Wickner PG, Saff R, Stone CA Jr., Robinson LB, Long AA, Wolfson AR, Williams P, Khan DA, Phillips E, and Blumenthal KG, mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J Allergy Clin Immunol Pract, 2021. 9(4): p. 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Povsic TJ, Lawrence MG, Lincoff AM, Mehran R, Rusconi CP, Zelenkofske SL, Huang Z, Sailstad J, Armstrong PW, Steg PG, Bode C, Becker RC, Alexander JH, Adkinson NF, Levinson AI, and Investigators R-P, Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J Allergy Clin Immunol, 2016. 138(6): p. 1712–1715. [DOI] [PubMed] [Google Scholar]
- 47.Dezsi L, Fulop T, Meszaros T, Szenasi G, Urbanics R, Vazsonyi C, Orfi E, Rosivall L, Nemes R, Kok RJ, Metselaar JM, Storm G, and Szebeni J, Features of complement activation-related pseudoallergy to liposomes with different surface charge and PEGylation: comparison of the porcine and rat responses. J Control Release, 2014. 195: p. 2–10. [DOI] [PubMed] [Google Scholar]
- 48.Zhou ZH, Stone CA Jr., Jakubovic B, Phillips EJ, Sussman G, Park J, Hoang U, Kirshner SL, Levin R, and Kozlowski S, Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract, 2021. 9(4): p. 1731–1733 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozma GT, Meszaros T, Vashegyi I, Fulop T, Orfi E, Dezsi L, Rosivall L, Bavli Y, Urbanics R, Mollnes TE, Barenholz Y, and Szebeni J, Pseudo-anaphylaxis to Polyethylene Glycol (PEG)-Coated Liposomes: Roles of Anti-PEG IgM and Complement Activation in a Porcine Model of Human Infusion Reactions. ACS Nano, 2019. 13(8): p. 9315–9324. [DOI] [PubMed] [Google Scholar]
- 50.Ju Y, Lee WS, Pilkington EH, Kelly HG, Li S, Selva KJ, Wragg KM, Subbarao K, Nguyen THO, Rowntree LC, Allen LF, Bond K, Williamson DA, Truong NP, Plebanski M, Kedzierska K, Mahanty S, Chung AW, Caruso F, Wheatley AK, Juno JA, and Kent SJ, Anti-PEG Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine. ACS Nano, 2022. 16(8): p. 11769–11780. [DOI] [PubMed] [Google Scholar]
- 51.Carreno JM, Singh G, Tcheou J, Srivastava K, Gleason C, Muramatsu H, Desai P, Aberg JA, Miller RL, Study Group P, Pardi N, Simon V, and Krammer F, mRNA-1273 but not BNT162b2 induces antibodies against polyethylene glycol (PEG) contained in mRNA-based vaccine formulations. Vaccine, 2022. 40(42): p. 6114–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bavli Y, Chen BM, Gross G, Hershko A, Turjeman K, Roffler S, and Barenholz Y, Anti-PEG antibodies before and after a first dose of Comirnaty® (mRNA-LNP-based SARS-CoV-2 vaccine). J Control Release, 2023. 354: p. 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ju Y, Carreno JM, Simon V, Dawson K, Krammer F, and Kent SJ, Impact of anti-PEG antibodies induced by SARS-CoV-2 mRNA vaccines. Nat Rev Immunol, 2023. 23(3): p. 135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian Y, Gao Z, Wang N, Hu M, Ju Y, Li Q, Caruso F, Hao J, and Cui J, Engineering Poly(ethylene glycol) Nanoparticles for Accelerated Blood Clearance Inhibition and Targeted Drug Delivery. Journal of the American Chemical Society, 2022. 144(40): p. 18419–18428. [DOI] [PubMed] [Google Scholar]
- 55.McSweeney MD, Price LSL, Wessler T, Ciociola EC, Herity LB, Piscitelli JA, DeWalle AC, Harris TN, Chan AKP, Saw RS, Hu P, Jennette JC, Forest MG, Cao Y, Montgomery SA, Zamboni WC, and Lai SK, Overcoming anti-PEG antibody mediated accelerated blood clearance of PEGylated liposomes by pre-infusion with high molecular weight free PEG. J Control Release, 2019. 311–312: p. 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mima Y, Hashimoto Y, Shimizu T, Kiwada H, and Ishida T, Anti-PEG IgM Is a Major Contributor to the Accelerated Blood Clearance of Polyethylene Glycol-Conjugated Protein. Molecular Pharmaceutics, 2015. 12(7): p. 2429–2435. [DOI] [PubMed] [Google Scholar]
- 57.Stidl R, Fuchs S, Bossard M, Siekmann J, Turecek PL, and Putz M, Safety of PEGylated recombinant human full-length coagulation factor VIII (BAX 855) in the overall context of PEG and PEG conjugates. Haemophilia, 2016. 22(1): p. 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffman RM, Yang Z, Tan Y, Han Q, Li S, and Yagi S, Safety and Toxicity of Recombinant Methioninase and Polyethylene Glycol (PEG) Recombinant Methioninase in Primates. Methods Mol Biol, 2019. 1866: p. 211–229. [DOI] [PubMed] [Google Scholar]
- 59.Webster R, Didier E, Harris P, Siegel N, Stadler J, Tilbury L, and Smith D, PEGylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug Metab Dispos, 2007. 35(1): p. 9–16. [DOI] [PubMed] [Google Scholar]
- 60.Berhanu AA, Krasnokutsky S, Keenan RT, and Pillinger MH, Pegloticase failure and a possible solution: Immunosuppression to prevent intolerance and inefficacy in patients with gout. Semin Arthritis Rheum, 2017. 46(6): p. 754–758. [DOI] [PubMed] [Google Scholar]
- 61.Bessen SY, Bessen MY, and Yung CM, Recapture and improved outcome of pegloticase response with methotrexate-A report of two cases and review of the literature. Semin Arthritis Rheum, 2019. 49(1): p. 56–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


