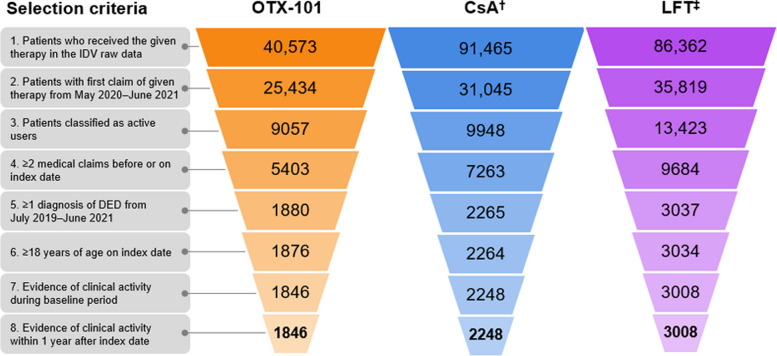
Fig. 2.
Study population selection. Note: Listed numerical values indicate the number of patients meeting the specified selection criterion for each step. †CsA cohort excludes patients who received OTX-101 at any point (n = 2971) or received LFT before CsA between May 2020 and June 2021 (n = 1465). ‡LFT cohort excludes patients who received OTX-101 at any point (n = 2380) or received CsA before LFT between May 2020 and June 2021 (n = 1753). CsA, cyclosporine ophthalmic emulsion 0.05%; DED, dry eye disease; IDV, Symphony Health Integrated Dataverse; LFT, lifitegrast ophthalmic solution 5%; OTX-101, cyclosporine ophthalmic solution 0.09%