Abstract
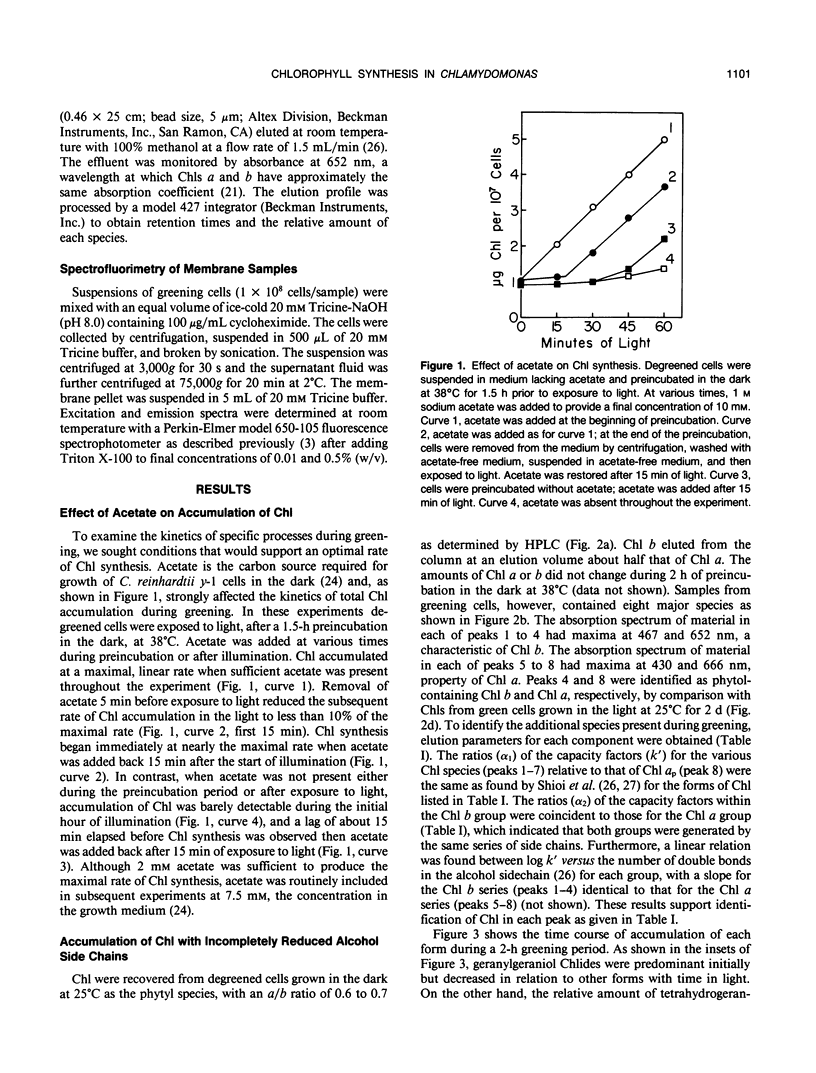
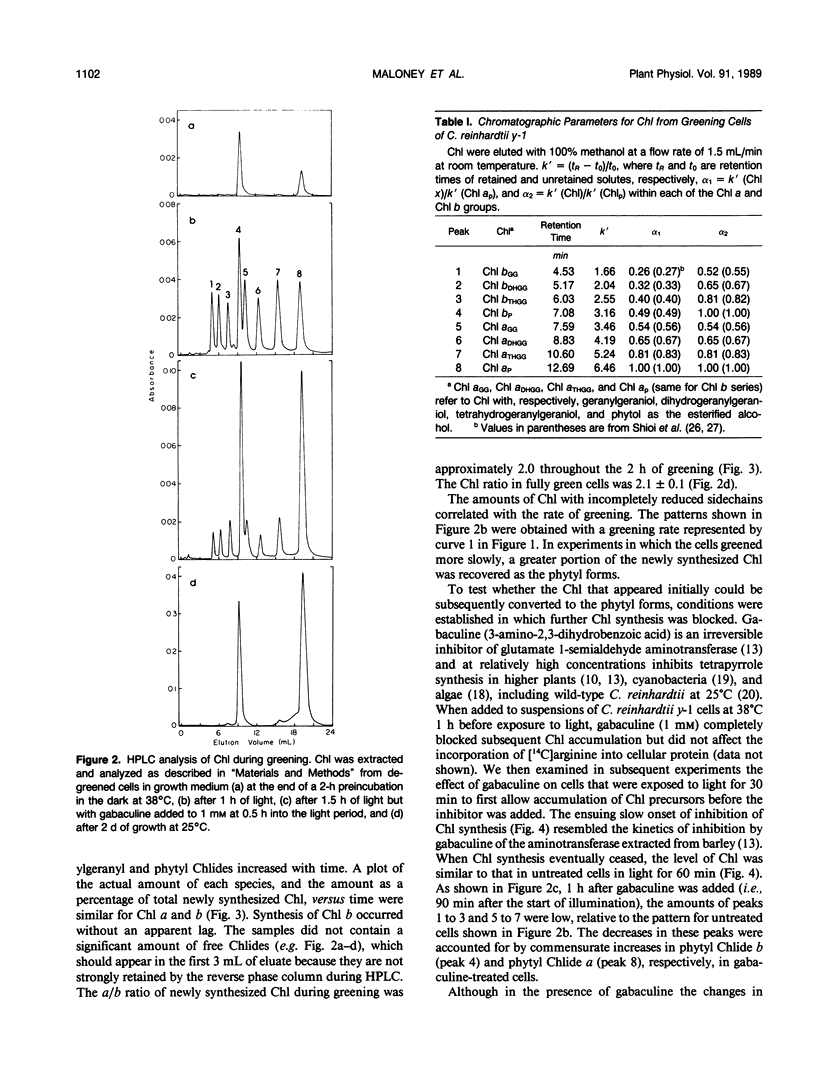
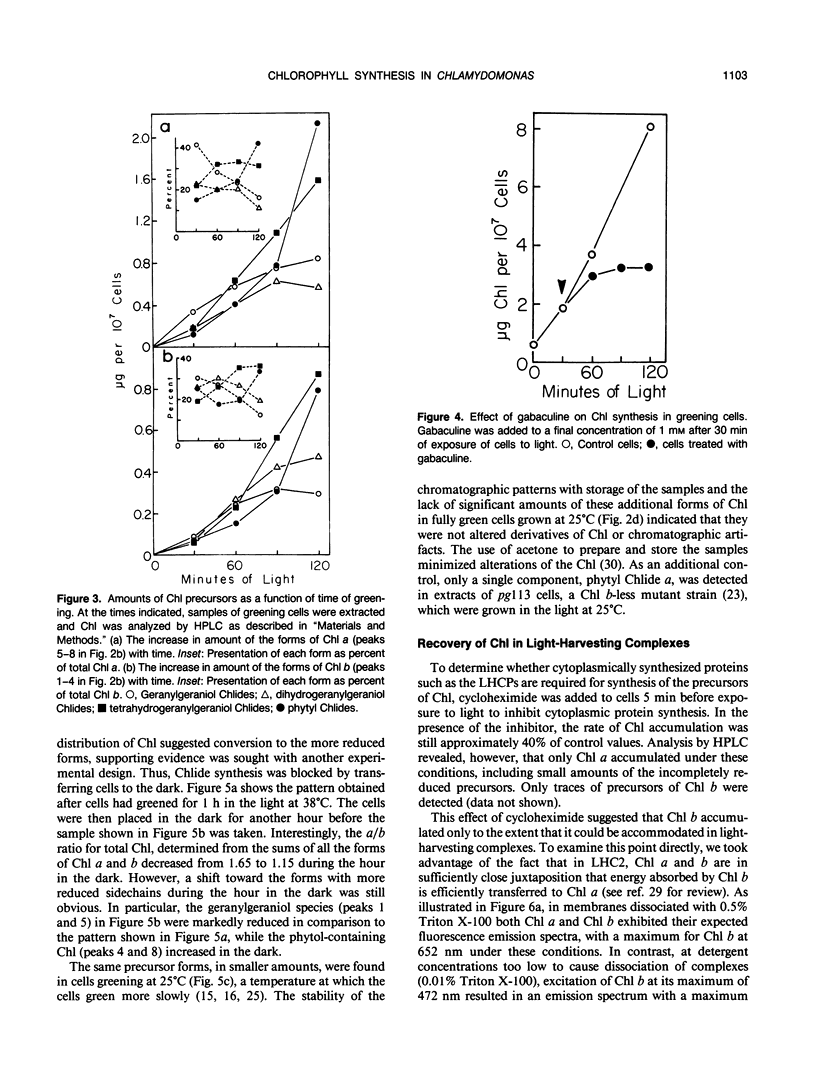
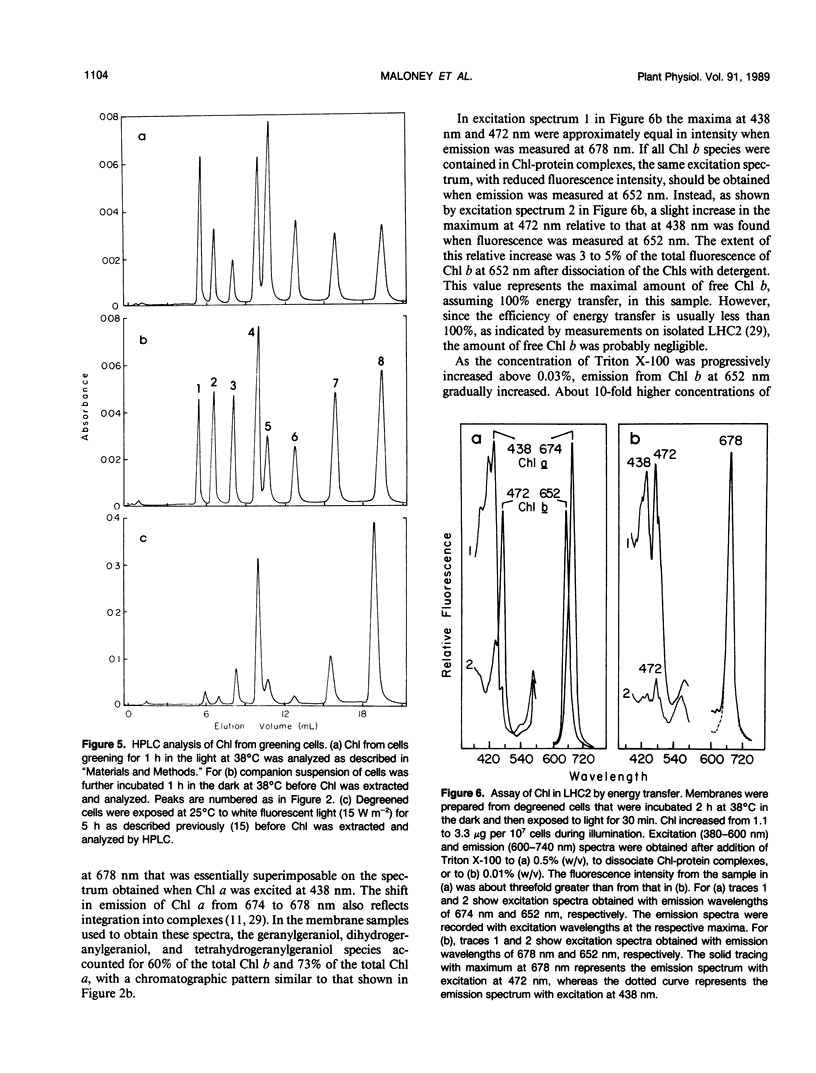
The initial kinetics of accumulation of chlorophylls (Chl) were analyzed during optimal greening of Chlamydomonas reinhardtii y-1 at 38°C. Acetate was required for maximal synthesis of Chl, which occurred at a linear rate when degreened cells were exposed to light. During the first hour Chl a and b accumulated predominantly as geranylgeraniol esters, with lesser amounts of the species with more reduced alcohol side chains. When Chl synthesis was blocked either by treatment with gabaculine or by transfer to the dark, the distribution shifted to the more reduced forms. Similar kinetic patterns indicated that a common pool of chlorophyllides a and b provided substrate for the enzymatic system that performs esterification and reduction of the sldechain for each group of Chl. Chl b was essentially quantitatively integrated into light-harvesting complexes as indicated by energy transfer to Chl a. In the presence of cycloheximide, an inhibitor of cytoplasmic protein synthesis, Chl b did not accumulate and Chl a production was reduced about one-half. The results demonstrate that Chl a/b-protein complexes assemble rapidly during greening and that reduction of the alcohol side chain of the Chl is not required for assembly of these complexes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarik D. P., Hoober J. K. Biosynthesis of a chlorophyllide b-like pigment in phenanthroline-treated Chlamydomonas reinhardtii y-1. Arch Biochem Biophys. 1985 Jul;240(1):369–379. doi: 10.1016/0003-9861(85)90042-6. [DOI] [PubMed] [Google Scholar]
- Bednarik D. P., Hoober J. K. Synthesis of Chlorophyllide b from Protochlorophyllide in Chlamydomonas reinhardtii y-1. Science. 1985 Oct 25;230(4724):450–453. doi: 10.1126/science.230.4724.450. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Kühlbrandt W. Determination of the aggregate size in detergent solution of the light-harvesting chlorophyll a/b-protein complex from chloroplast membranes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3797–3801. doi: 10.1073/pnas.85.11.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Electrophoretic purification of chlorophyll a/b-protein complexes from Chlamydomonas reinhardtii and spinach and analysis of their polypeptide compositions. J Biol Chem. 1981 Sep 10;256(17):9300–9307. [PubMed] [Google Scholar]
- Elich T. D., Lagarias J. C. Phytochrome Chromophore Biosynthesis : Both 5-Aminolevulinic Acid and Biliverdin Overcome Inhibition by Gabaculine in Etiolated Avena sativa L. Seedlings. Plant Physiol. 1987 Jun;84(2):304–310. doi: 10.1104/pp.84.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Ohad I. Chloroplast-cytoplasmic interrelations involved in chloroplast development in Chlamydomonas reinhardi y-1: effect of selective depletion of chloroplast translates. J Cell Biol. 1980 Oct;87(1):124–131. doi: 10.1083/jcb.87.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Kahn A., Ash D. E., Gough S., Kannangara C. G. Biosynthesis of delta-aminolevulinate in greening barley leaves. IX. Structure of the substrate, mode of gabaculine inhibition, and the catalytic mechanism of glutamate 1-semialdehyde aminotransferase. Carlsberg Res Commun. 1988;53(1):11–25. doi: 10.1007/BF02908411. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Marks D. B., Keller B. J., Margulies M. M. Regulation of accumulation of the major thylakoid polypeptides in Chlamydomonas reinhardtii y-1 at 25 degrees C and 38 degrees C. J Cell Biol. 1982 Nov;95(2 Pt 1):552–558. doi: 10.1083/jcb.95.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Stegeman W. J. Control of the synthesis of a major polypeptide of chloroplast membranes in Chlamydomonas reinhardi. J Cell Biol. 1973 Jan;56(1):1–12. doi: 10.1083/jcb.56.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Stegeman W. J. Kinetics and regulation of synthesis of the major polypeptides of thylakoid membranes in Chlamydomonas reinhardtii y-1 at elevated temperatures. J Cell Biol. 1976 Aug;70(2 Pt 1):326–337. doi: 10.1083/jcb.70.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. A., Thompson W. F., Briggs W. R. Phytochrome Regulation of Greening in Pisum: Chlorophyll Accumulation and Abundance of mRNA for the Light-Harvesting Chlorophyll a/b Binding Proteins. Plant Physiol. 1988 Jan;86(1):299–305. doi: 10.1104/pp.86.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J. D., Turner L., Brown S. B. The effect of gabaculine on tetrapyrrole biosynthesis and heterotrophic growth in Cyanidium caldarium. Biochem J. 1988 Sep 15;254(3):907–910. doi: 10.1042/bj2540907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnoë P., Mayfield S. P., Rochaix J. D. Comparative analysis of the biogenesis of photosystem II in the wild-type and Y-1 mutant of Chlamydomonas reinhardtii. J Cell Biol. 1988 Mar;106(3):609–616. doi: 10.1083/jcb.106.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. I. Plastid dedifferentiation in a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):521–552. doi: 10.1083/jcb.35.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):553–584. doi: 10.1083/jcb.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Schultz G., Rüdiger W., Benz J. Hydrogenation of geranylgeraniol : two pathways exist in spinach chloroplasts. Plant Physiol. 1983 Apr;71(4):849–854. doi: 10.1104/pp.71.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]


