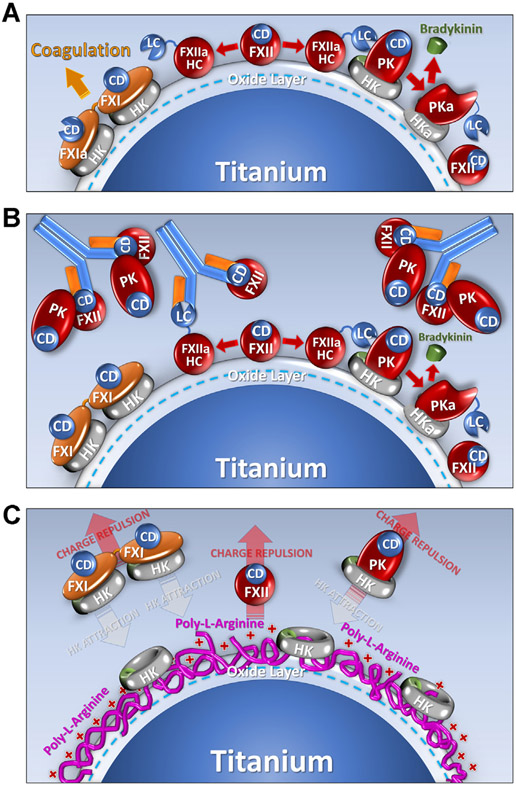
FIGURE 3.
Contact activation on titanium. Shown are diagrams of proposed mechanisms by which a titanium (Ti) surface supports contact activation, and the effects of the anti-FXII antibody 5C12, or poly-L-arginine surface coating on the process. The Ti surface is assumed to be covered with a layer of TiO2 that carries a negative surface charge at physiologic pH. (A). Contact Activation. When plasma is exposed to Ti the protease zymogens factor XII (FXII), factor XI (FXI), and prekallikrein (PK) bind to the Ti. For PK and FXII, surface binding is enhanced by the cofactor high-molecular-weight-kininogen (HK). The abbreviation CD indicates the catalytic domain of the zymogens, and HC (heavy chain) and LC (light chain) are the 2 parts of the activated proteases FXIIa, FXIa, and plasma kallikrein (PKa). During contact activation, FXII undergoes autoactivation to FXIIa, which converts PK to PKa. PKa, in turn, reciprocally activates FXII to FXIIa. FXIIa converts FXI to FXIa to promote thrombin generation and blood coagulation. PKa cleaves HK to release the vasoactive peptide bradykinin. (B). Effect of IgG 5C12. 5C12 binds to the catalytic domain of FXII and FXIIa blocking catalytic activity, which limits downstream activation of FXI and PK, blocking clotting and bradykinin formation. 5C12 also seems to displace FXII from the Ti surface. Interestingly, PK also is displaced from the surface by 5C12, suggesting that some PK binds to the Ti surface through an interaction with FXII. (C). Effect of poly-L-arginine coating of Ti. Poly-L-arginine appears to avidly bind to the Ti surface and prevents access to binding sites for FXII, PK, and FXI. It may also repel FXII, FXI, and PK from the surface. Interestingly, HK can bind to poly-L-arginine coated Ti, but it seems to not be associated with PK or FXI in this situation. Nearly, all FXI and most PK circulates in plasma in a complex with HK. These complexes apparently do not bind well to poly-L-arginine (perhaps due to repulsion of FXI and PK).