Figure 3. . Profiling of CD33D2 cell surface expression on primary AML cells obtained at diagnosis.
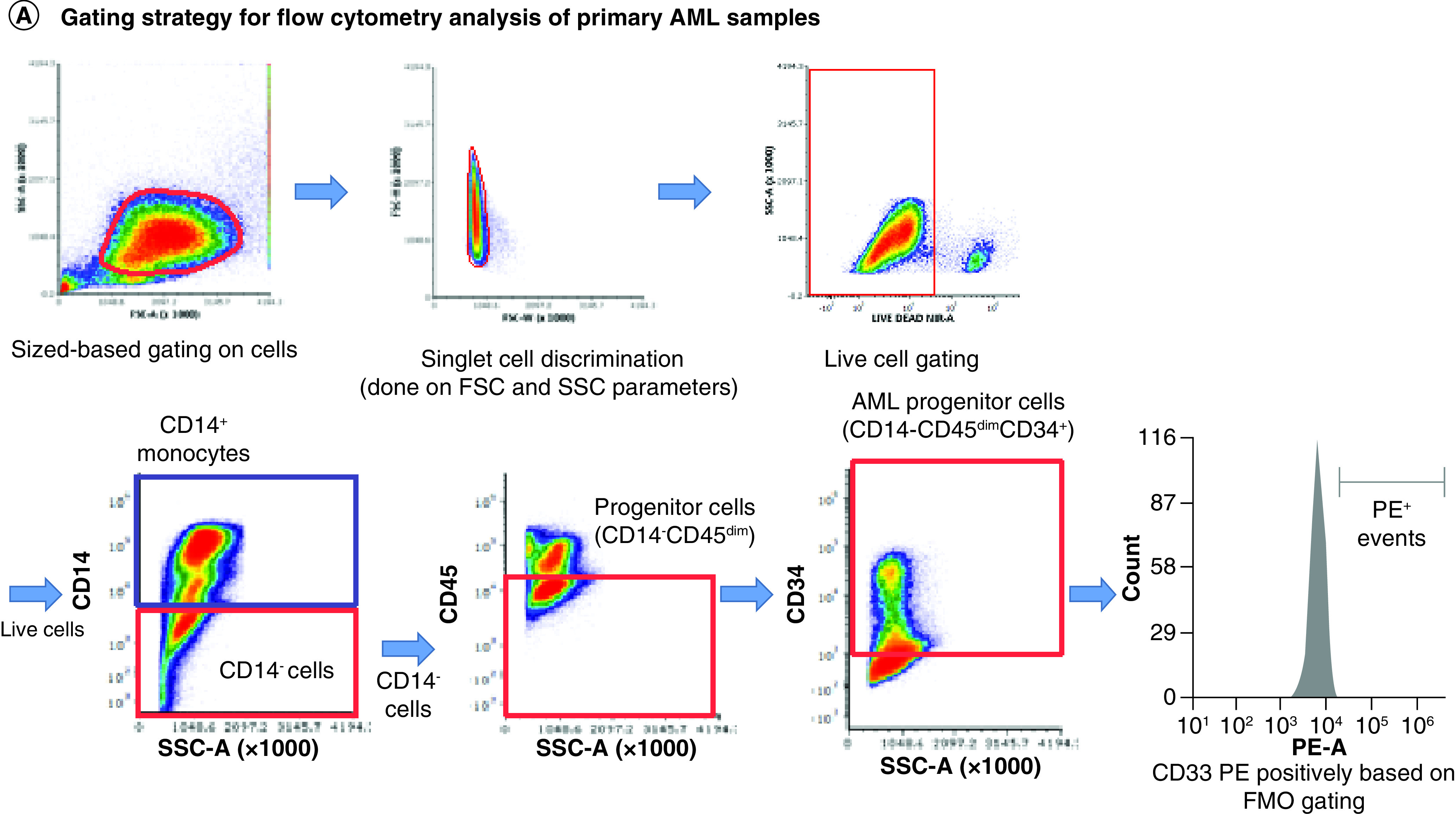
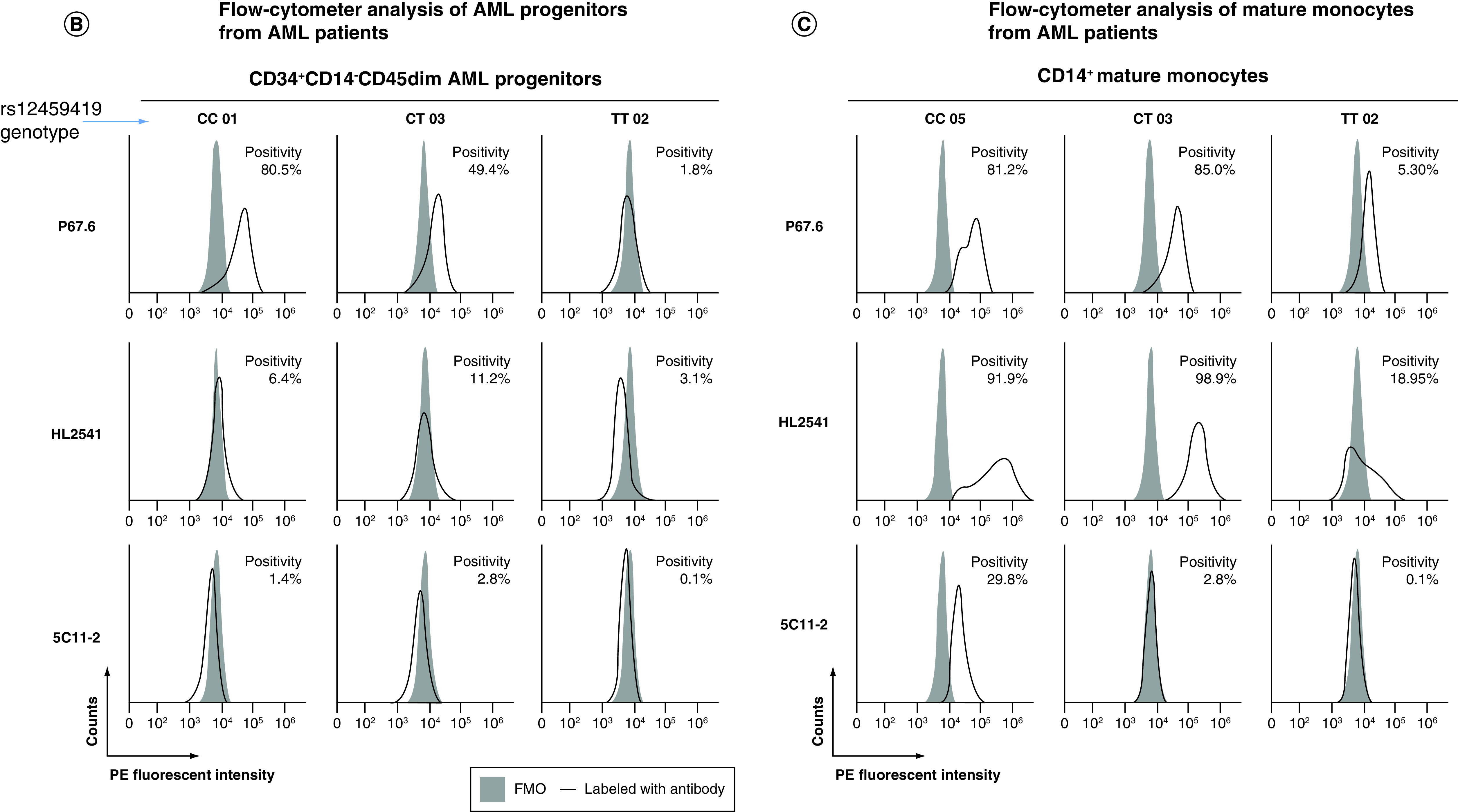
(A) Gating strategy for analysis of CD33D2 expression on CD34+CD14-CD45dim AML progenitors, CD14+ mature monocytes and other CD45high lymphocytes using CD34-APC (100 ng), CD14-pacific blue (800 ng) and CD45-super bright 600 antibodies (150 ng). Cells were first gated by size and viability. Subsequently, CD14- cells were gated, followed by a gate set to capture CD14-CD45dim and CD14-CD45high (other lymphocytes) and finally a gate to capture CD14-CD45dimCD34+ cells (AML progenitors). Following gating based on surface marker antibodies, a gate for positive fluorescence in the PE channel was set using FMO controls as negatives. All gates were set using unstained samples as a negative. (B) Staining for CD33D2 using HL2541-PE and 5C11-2-PE (500 ng each) on CD34+CD14-CD45dim AML progenitors from primary BM AML specimens of different rs12459419 genotypes. (C) Staining for CD33D2 using HL2541-PE and 5C11-2-PE on CD14+ mature monocytes from primary BM AML specimens of different rs12459419 genotypes. All flow cytometry data were collected on the Cytek Aurora platform and analyzed using FCS Express. MFI ratios were calculated as follows: (sample MFI/FMO MFI). Percent positivity was calculated based on marker positivity, as shown in the gating strategies (Supplementary Figures 1A & 3A). All figures are representative of N ≥3 experiments.
AML: Acute myeloid leukemia; BM: Bone marrow; FMO: Fluorescence minus one; MFI: Median fluorescence intensity; PE: Phycoerythrin.
