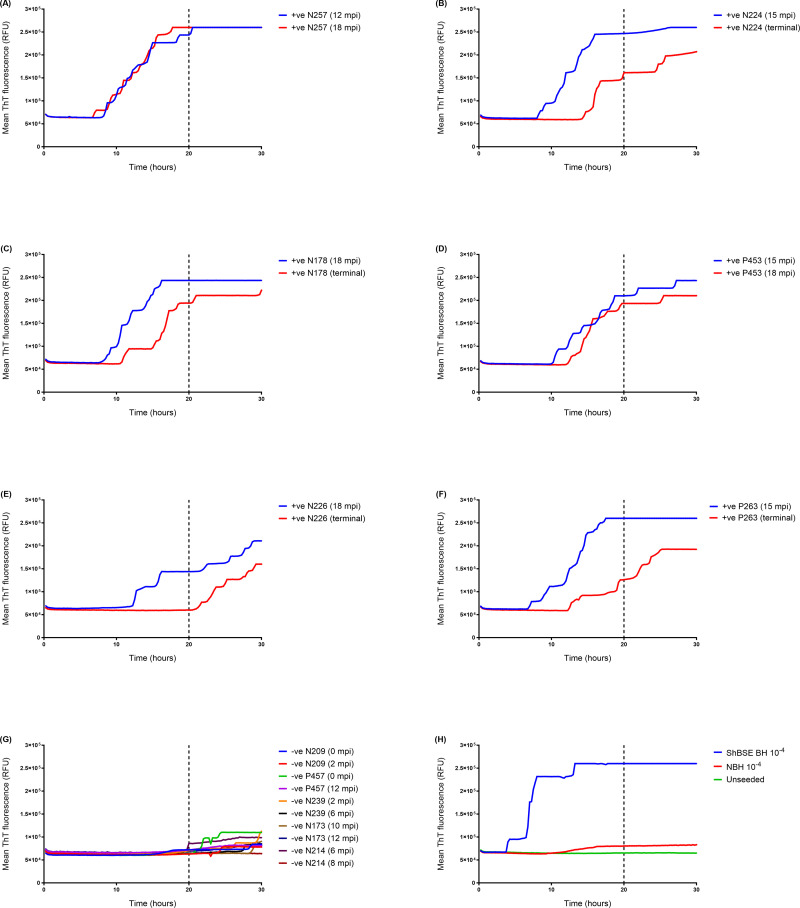
Fig 5. WB IOME RT-QuIC analysis of a panel of blood samples from BSE-infected sheep (endogenous infectivity).
Samples of whole blood (n = 12) from BSE positive (+ve) sheep collected at different time points (as indicated on figure) were tested by WB IOME RT-QuIC in three independent experiments (equivalent to a total of n = 12 replicate reactions/sample). Blood samples from BSE-infected donor sheep (A) N257, (C) N178, and (E) N226 were tested alongside samples from BSE-infected recipients (B) N224, (D) P453, and (F) P263. Samples were considered positive if ≥ 50% (6/12) replicate reactions exceeded threshold fluorescence within a designated cut-off time of 20 h (as indicated by vertical dotted lines). (G) Whole blood samples (n = 10) from mock-infected, negative (-ve) control sheep were run alongside. A total of n = 128 negative control reactions were tested; samples N209 (2 mpi), P457 (12 mpi), N239 (6 mpi), N173 (12 mpi), N214 (6 mpi) and N214 (8 mpi) were tested in three independent experiments (equivalent to n = 12 replicate reactions/sample); samples N209 (0 mpi), P457 (0 mpi) and N239 (2 mpi) were tested in four independent experiments (n = 16 replicate reactions/sample); and sample N173 (10 mpi) was tested in two independent experiments (n = 8 replicate reactions).