Abstract
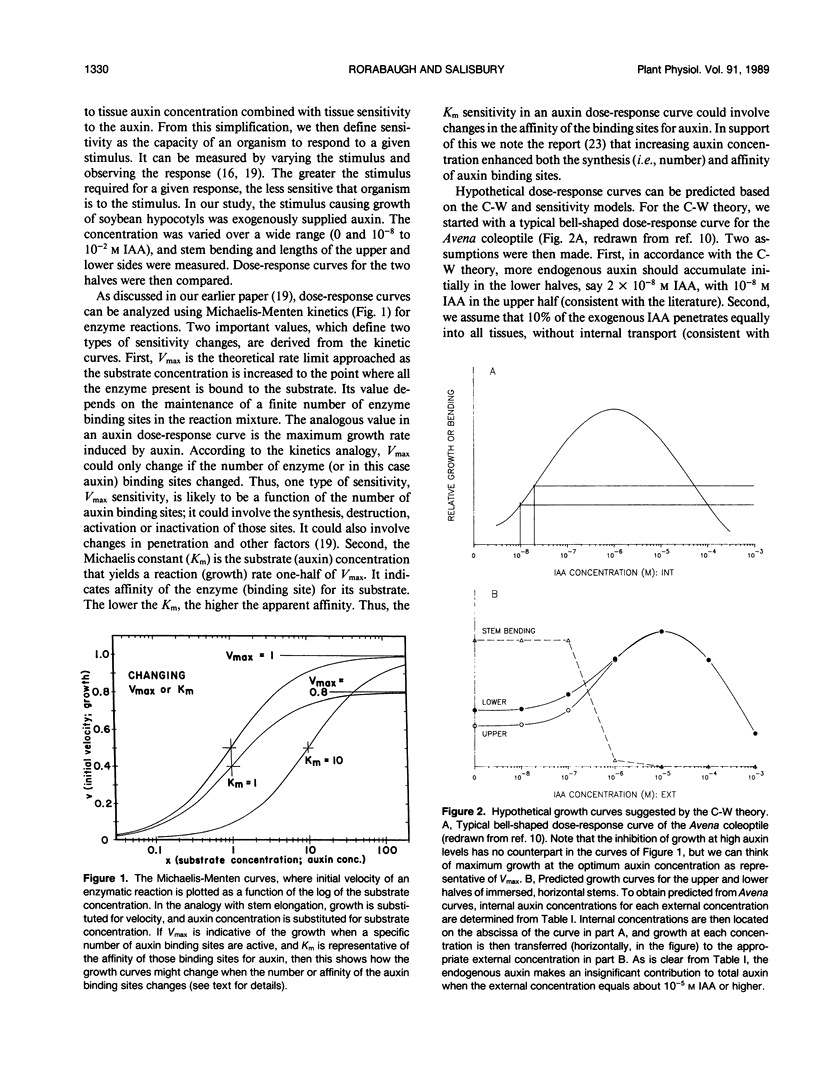
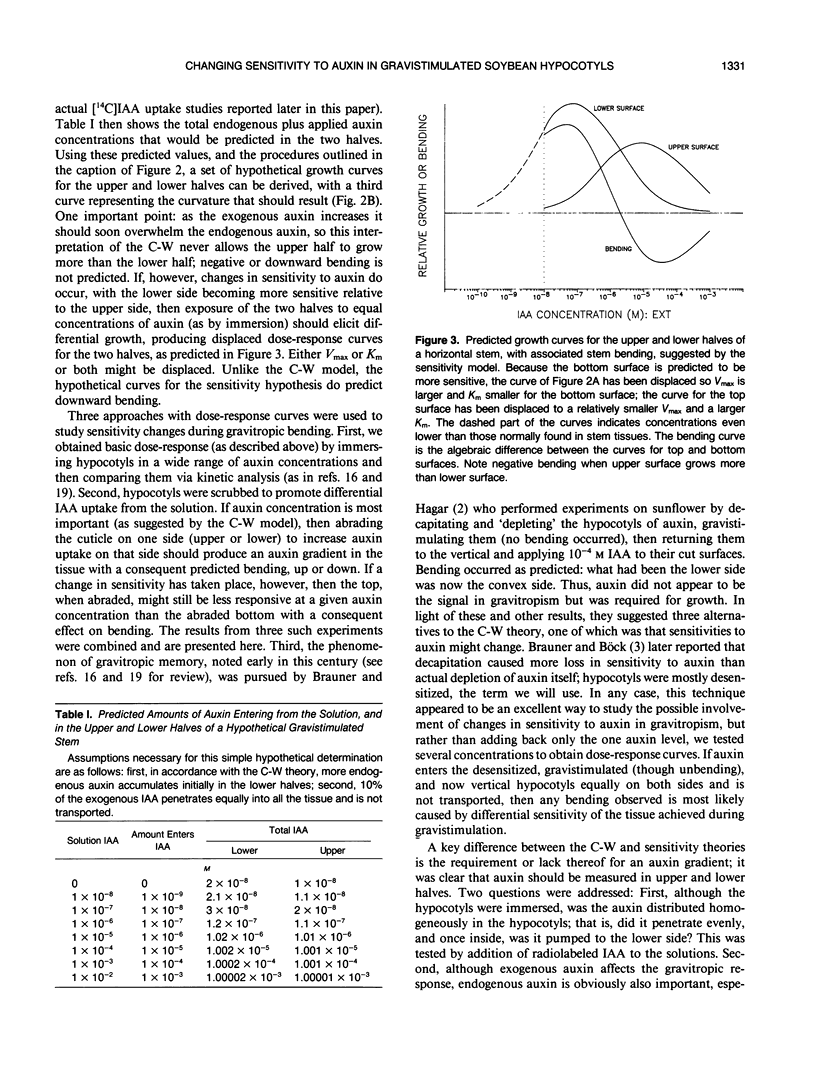
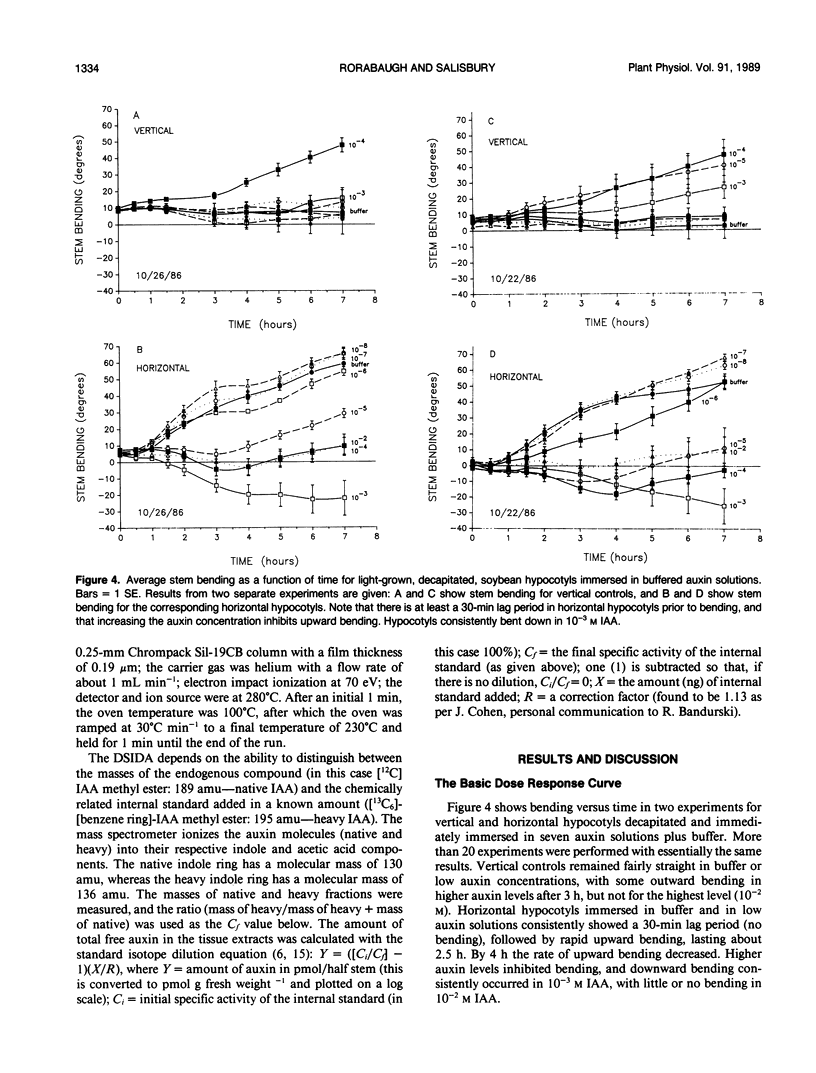
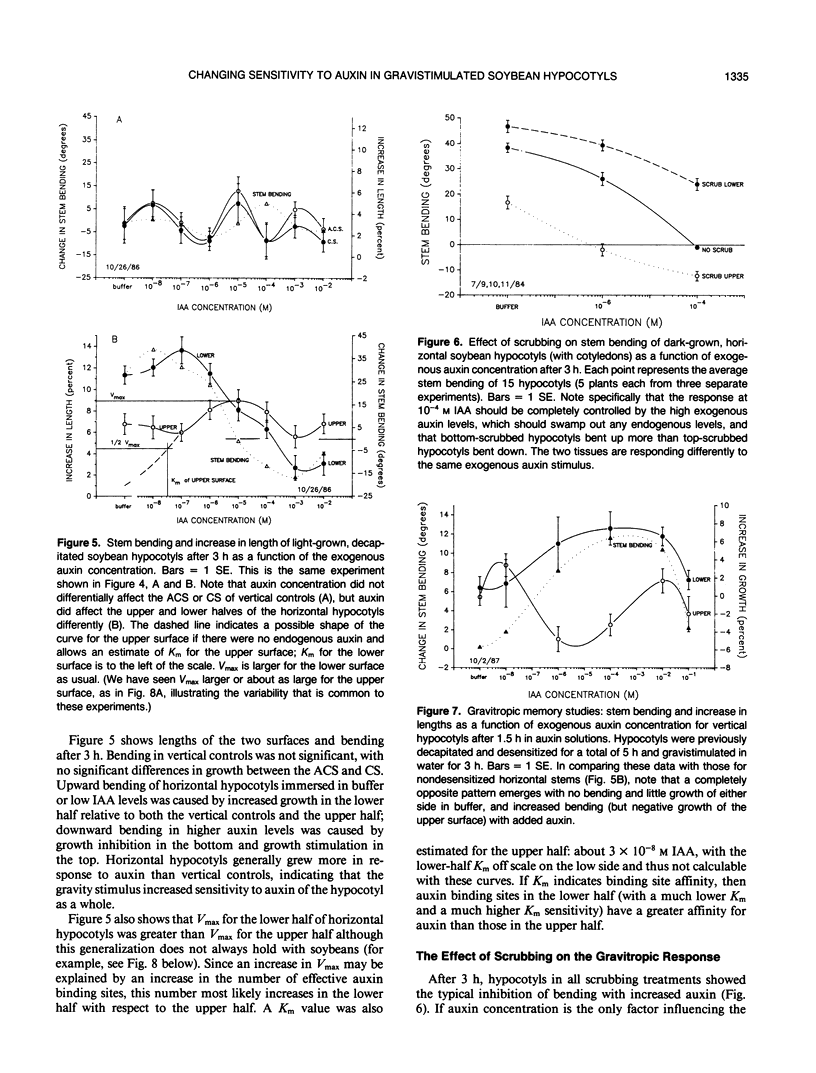
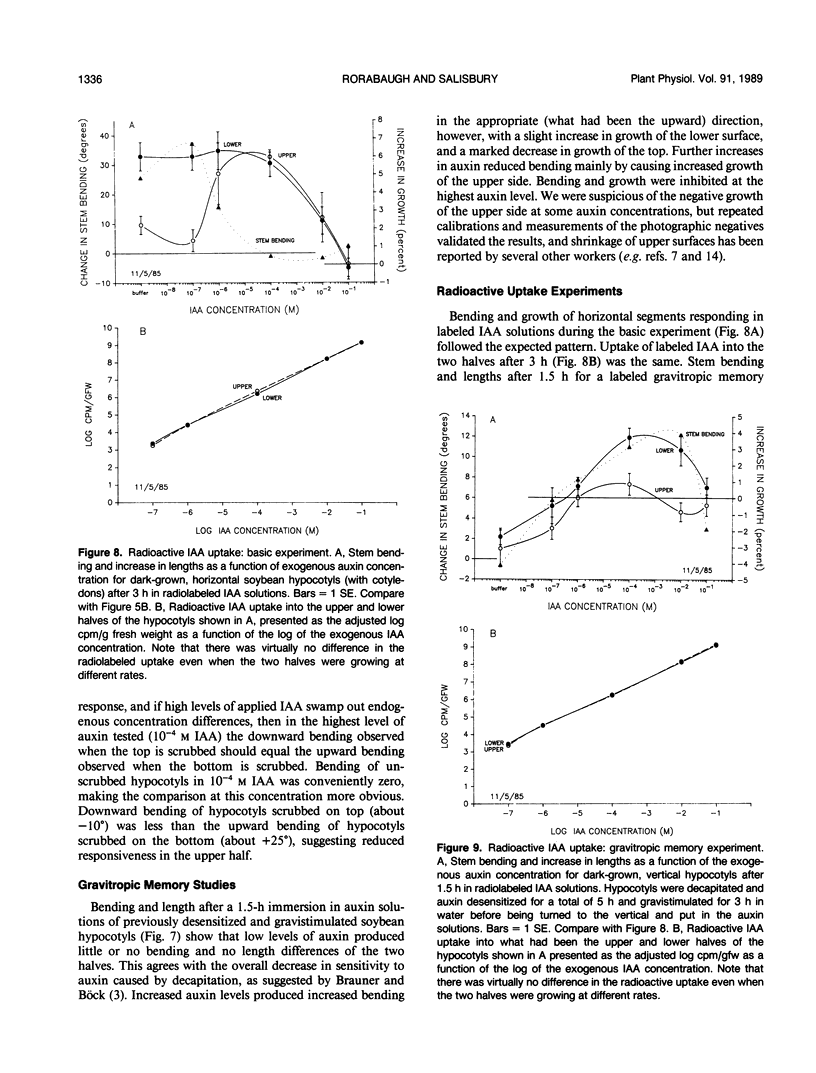
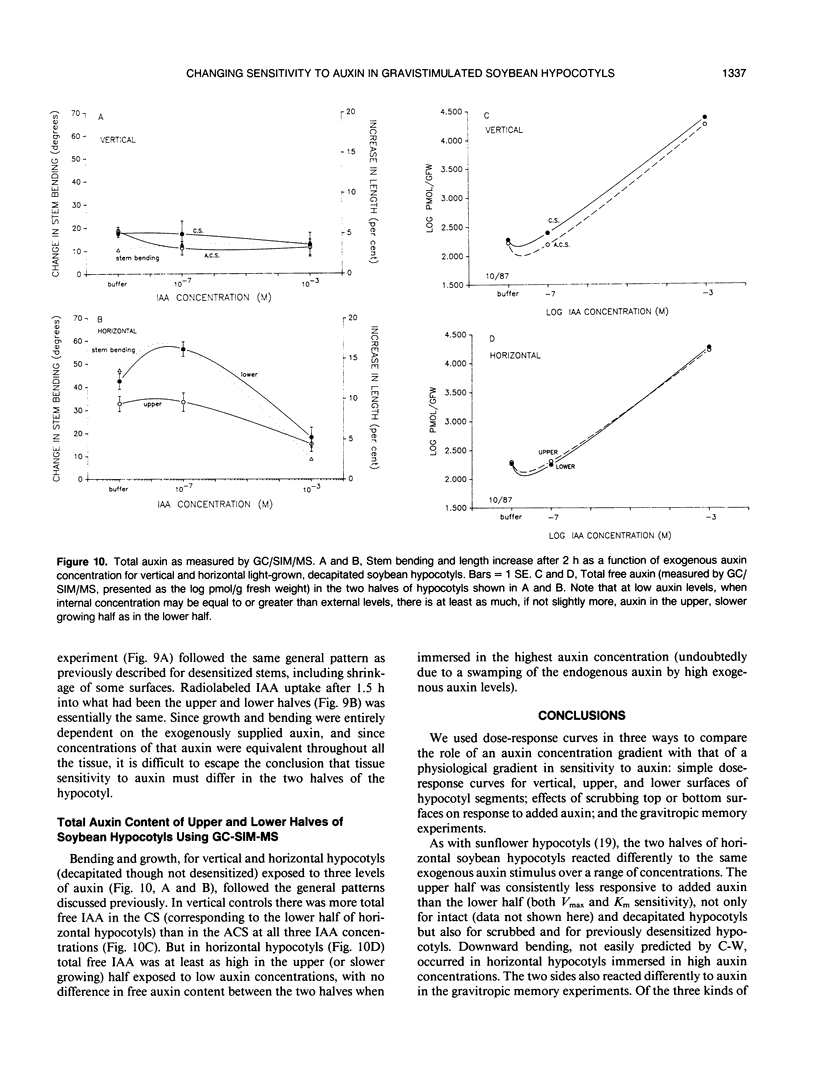
Although the Cholodny-Went model of auxin redistribution has been used to explain the transduction phase of gravitropism for over 60 years, problems are apparent, especially with dicot stems. An alternative to an auxin gradient is a physiological gradient in which lower tissues of a horizontal stem become more sensitive than upper tissues to auxin already present. Changes in tissue sensitivity to auxin were tested by immersing marked Glycine max Merrill (soybean) hypocotyl sections in buffered auxin solutions (0, 10−8 to 10−2 molar indoleacetic acid) and observing bending and growth of upper and lower surfaces. The two surfaces of horizontal hypocotyl sections responded differently to the same applied auxin stimulus; hypocotyls bent up (lower half grew more) in buffer alone or in low auxin levels, but bent down (upper half grew more) in high auxin. Dose-response curves were evaluated with Michaelis-Menten kinetics, with auxin-receptor binding analogous to enzyme-substrate binding. Vmax for the lower half was usually greater than that for the upper half, which could indicate more binding sites in the lower half. Km of the upper half was always greater than that of the lower half (unmeasurably low), which could indicate that upper-half binding sites had a much lower affinity for auxin than lower-half sites. Dose-response curves were also obtained for sections `scrubbed' (cuticle abraded) on top or bottom before immersion in auxin, and `gravitropic memory' experiments of L. Brauner and A. Hagar (1958 Planta 51: 115-147) were duplicated. [1-14C]Indoleacetic acid penetration was equal into the two halves, and endogenous plus exogenously supplied (not radiolabeled) free auxin in the two halves (by gas chromatography-selected ion monitoring-mass spectrometry) was also equal. Thus, differential growth occurred without free auxin redistribution, contrary to Cholodny-Went but in agreement with a sensitivity model.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkman T., Leopold A. C. An electric current associated with gravity sensing in maize roots. Plant Physiol. 1987;84:841–846. doi: 10.1104/pp.84.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S., Chabot J. F., Morrison G. H., Leopold A. C. Localization of calcium in amyloplasts of root-cap cells using ion microscopy. Science. 1982 Jun 11;216(4551):1221–1223. doi: 10.1126/science.216.4551.1221. [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Schulze A. Double-standard isotope dilution assay. I. Quantitative assay of indole-3-acetic acid. Anal Biochem. 1981 Apr;112(2):249–257. doi: 10.1016/0003-2697(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Dowler M. J., Rayle D. L. Auxin Does Not Alter the Permeability of Pea Segments to Tritium-labeled Water. Plant Physiol. 1974 Feb;53(2):229–232. doi: 10.1104/pp.53.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. J., McRae D. H., Bonner J. Auxin-Induced Growth Inhibition a Natural Consequence of Two-Point Attachment. Proc Natl Acad Sci U S A. 1952 Dec;38(12):1014–1022. doi: 10.1073/pnas.38.12.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- Macdonald I. R., Hart J. W. New light on the cholodny-went theory. Plant Physiol. 1987 Jul;84(3):568–570. doi: 10.1104/pp.84.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller W. J., Salisbury F. B., Blotter P. T. Gravitropism in Higher Plant Shoots : II. Dimensional and Pressure Changes during Stem Bending. Plant Physiol. 1984 Dec;76(4):993–999. doi: 10.1104/pp.76.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack F. D., Suyemoto M. M., Leopold A. C. Amyloplast sedimentation and organelle saltation in living corn columella cells. Am J Bot. 1986 Dec;73(12):1692–1698. [PubMed] [Google Scholar]
- Salisbury F. B., Gillespie L., Rorabaugh P. Gravitropism in higher plant shoots. V. Changing sensitivity to auxin. Plant Physiol. 1988;88:1186–1194. doi: 10.1104/pp.88.4.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]


