In recognition that race is a social and not a biological construct, the National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) Task Force recommend the immediate discontinuation of estimated glomerular filtration rate (eGFR) equations containing a race correction factor for Black people1, and implement the revised Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR equation2. Most health systems are moving forward or have already implemented these equations3.
The National Cardiovascular Data Registry (NCDR) acute kidney injury (AKI) risk model4 (‘NCDR model’), estimates AKI risk with percutaneous coronary intervention (PCI) to inform decision making and management and a reduced eGFR is the strongest predictor. However, the NCDR model and our subsequent tree-based machine learning model (‘ML model’)5, were derived using GFR estimated by the Modification of Diet in Renal Disease Study (MDRD) equation. While the new CKD-EPI equation was shown to reverse the overestimation of GFR for Black patients, it remains unknown how the new equation affects AKI model performance for both Black and non-Black patients. Accordingly, we assessed the effect of incorporating the new CKD-EPI equation on AKI risk estimates for Black and non-Black people for both the NCDR model4 and ML model5.
We used the same data used to develop the original NCDR and ML models – all PCIs between June 1, 2009 and June 30, 2011. AKI was defined as an increase in creatinine ≥ 0.3 mg/dL or 50% from baseline. On a random 70% of the cohort (training set), we first duplicated the NCDR and ML models and then trained corresponding models with the new CKD-EPI equation. Evaluated on the remaining 30% cohort (test set), we reported model calibration by comparing the average predicted AKI risk with the observed AKI rate for Black and non-Black patients. We also compared discrimination performance via c-statistics. We did not expect the c-statistic to change as the new eGFR equation mainly resulted in calibration change.2 Confidence intervals were estimated by bootstrapping. Performance comparison was evaluated by paired t-test. The models were also tested on a more contemporary cohort including procedures from July 1, 2011 to June 30, 2017. The Yale University Institutional Review Board exempted this study from individual consent. R (version 3.6.0) was used for analysis. Because of the sensitive nature of the data collected for this study, requests to access the dataset may be sent to ACC NCDR at cvquality.acc.org.
The cohort included 947,091 PCIs and 7.9% patients were Black. The AKI rate was 10.2% and 7.1% in Black and non-Black patients, respectively. The difference between predicted versus observed AKI rates in the test set stratified by race is shown in Figure 1. The original NCDR model significantly underestimated risk in Black patients (difference [95% confidence interval [CI]] −2.6% [−2.7%, −2.5%]) and overestimated risk in non-Black patients (difference [95% CI] 0.3% [0.2%, 0.3%]). The revised CKD-EPI equation reduced the underestimation among Black patients (difference −2.1% vs −2.6, p<0.001) and overestimation among non-Black patients (difference 0.2% vs 0.3%, p<0.001).
Figure 1:
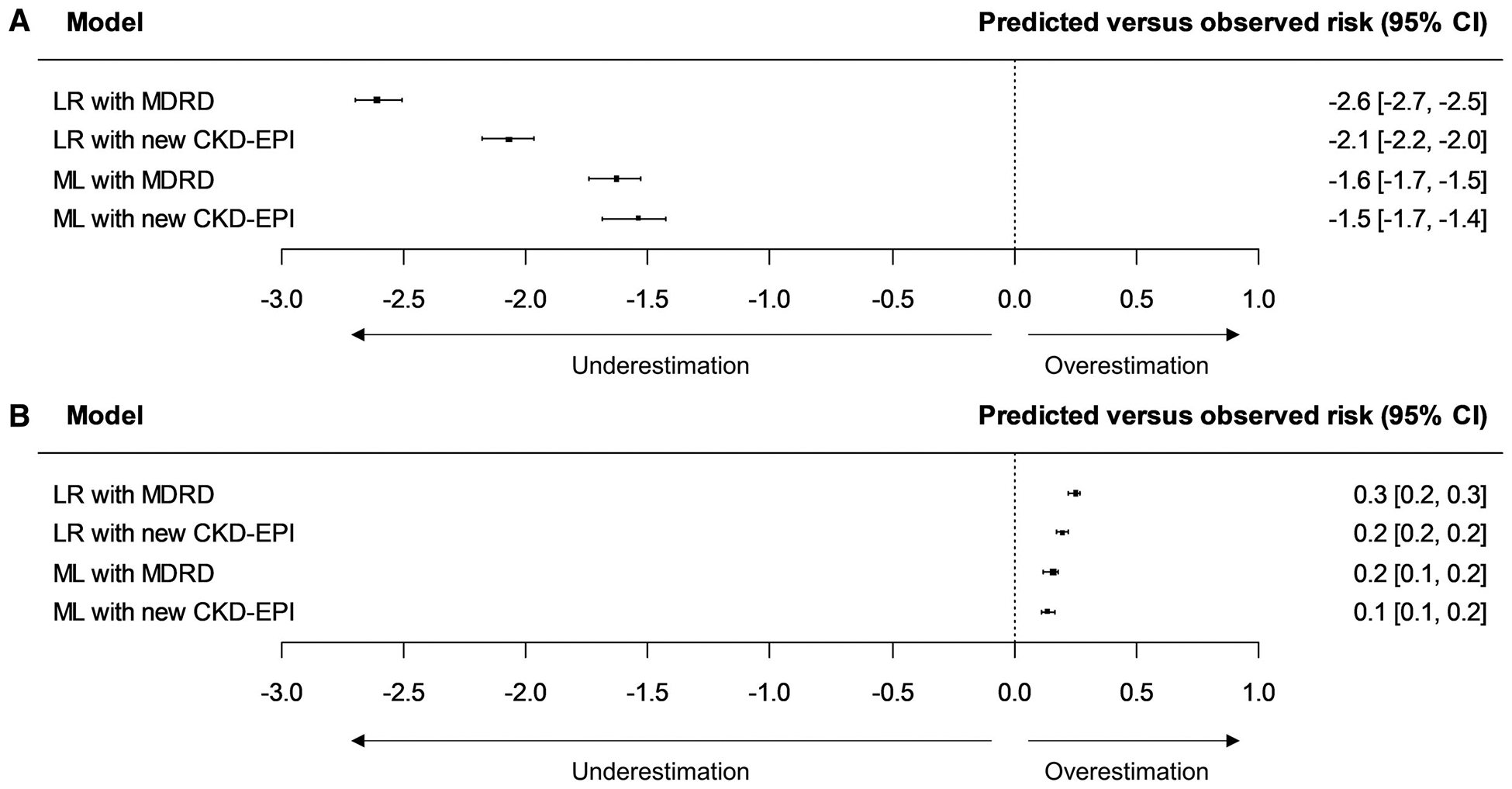
Model calibration performance comparison. A) Calibration performance of the National Cardiovascular Data Registry (NCDR) logistic regression (LR) and machine learning (ML) model with the original Modification of Diet in Renal Disease Study (MDRD) and new Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in Black patients; B) Calibration performance of the models in non-Black patients. CI: confidence interval.
Similarly, the original ML model underestimated AKI risks for Black patients, but to a lesser degree (difference [95% CI] −1.6% [−1.7%, −1.5%]). The incorporation of the CKD-EPI equation improved the calibration for Black (difference −1.5% vs −1.6%, p<0.001) and non-Black patients (difference 0.1% vs 0.2%, p<0.001). There was minimal change in c-statistic and the results on the contemporary cohort were similar (results not shown).
Our study shows that the revised creatinine-based CKD-EPI equation, which eliminates the race correction factor, improves post-PCI AKI risk prediction for Black patients and non-Black patients. The revised CKD-EPI equation has been shown to eliminate the overestimation for Black patients (a median of 3.7 ml/dL) but underestimate it by 3.6 ml/dL. In contrast, the new equation for non-Black patients increases the overestimation from a median of 0.5 ml/dL to 3.9 ml/dL.2 The original AKI models had significantly underestimated the risk for Black patients and slightly overestimated the risk for non-Black patients. The revised CKD-EPI equation improved risk estimation for both groups.
Although the removal of the race correction factor in eGFR calculation improves current risk models to some degree, self-identified Black patients remain at much higher risk for AKI after PCI than predicted. The severity of the current predictors in the model such as diabetes may not be adequately captured and the current predictors may be insufficient and novel risk factors need to be studied. Also, there is a need to disentangle biologic risk factors that may correlate with social factors. Limitations include the outcome being in-hospital events and NCDR not being representative of patients in non-US centers.
The revised CKD-EPI equation improves post-PCI AKI risk stratification for Black and non-Black patients, further supporting its immediate implementation. However, the systematic underestimation of risks for Black patients calls for the collection of richer data capture determinants of this elevated risk.
Sources of Funding:
Dr. Masoudi had a contract with the American College of Cardiology for his role as Chief Scientific Advisor, National Cardiovascular Data Registry. Dr Mortazavi reported receiving grants from the National Institute of Biomedical Imaging and Bioengineering, National Heart, Lung, and Blood Institute, United States Food and Drug Administration, the National Science Foundation, and the United States Department of Defense Advanced Research Projects Agency outside the submitted work. Dr. Mortazavi has a pending patent (US20180315507A1) and is a technical consultant for Hugo Health. In the past 3 years, Dr. Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Reality Labs, Tesseract/4Catalyst, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, and F-Prime. He is a co-founder of Refactor Health and HugoHealth, and is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare & Medicaid Services and through Yale University from Johnson & Johnson. Other authors had no external funding.
REFERENCES
- 1.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey-Mims MM, Roberts GV, et al. A unifying approach for gfr estimation: Recommendations of the nkf-asn task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2021;79:268–288 [DOI] [PubMed] [Google Scholar]
- 2.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. New creatinine- and cystatin c–based equations to estimate gfr without race. N Engl J Med. 2021;385:1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genzen JR, Souers RJ, Pearson LN, Manthei DM, Chambliss AB, Shajani-Yi Z, Miller WG. Reported awareness and adoption of 2021 estimated glomerular filtration rate equations among us clinical laboratories, march 2022. JAMA. 2022;328:2060–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Weintraub WS, Curtis JP, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the national cardiovascular data registry cath-pci registry. J Am Heart Assoc. 2014;3:e001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Murugiah K, Mahajan S, Li SX, Dhruva SS, Haimovich JS, Wang Y, Schulz WL, Testani JM, Wilson FP, et al. Enhancing the prediction of acute kidney injury risk after percutaneous coronary intervention using machine learning techniques: A retrospective cohort study. PLoS Med. 2018;15:e1002703. [DOI] [PMC free article] [PubMed] [Google Scholar]


