Abstract
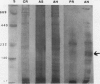
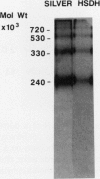
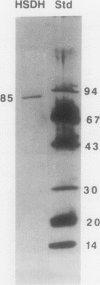
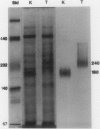
Homoserine dehydrogenase from cell suspension cultures of carrot (Daucus carota L.) has been purified to apparent homogeneity by a combination of selective heat denaturation, ion exchange and gel filtration chromatographies, and preparative gel electrophoresis. Carrot homoserine dehydrogenase is composed of subunits of equal molecular weight (85,000 ± 5,000). During purification, the enzyme exists predominantly in two molecular weight forms, 180,000 and 240,000. The enzyme can be reversibly converted from one form to the other, and each has different regulatory properties. When the enzyme is dialyzed in the presence of 5 millimolar threonine, the purified enzyme is converted into its trimeric form (240,000), which is completely inhibited by 5 millimolar threonine and is stimulated 2.6-fold by K+. When the enzyme is dialyzed in the presence of K+ and absence of threonine, the purified enzyme is converted into a dimer (180,000), which is not inhibited by threonine and is only stimulated 1.5-fold by K+. The enzyme also can polymerize under certain conditions to form higher molecular weight aggregates ranging in size up to 720,000, which also are catalytically active. This interconversion of homoserine dehydrogenase conformations may reflect the daily stream of events occurring in vivo. When light stimulates protein synthesis, the threonine pool decreases in the chloroplast, while K+ concentrations increase. The change in threonine and K+ concentrations shift the homoserine dehydrogenase from the threonine-sensitive to the threonine-insensitive conformation resulting in increased production of threonine, which would meet the demands of protein synthesis. The reverse process would occur in the dark.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan J. K., Lissik E. A., Matthews B. F. Changes in Enzyme Regulation during Growth of Maize: III. Intracellular Localization of Homoserine Dehydrogenase in Chloroplasts. Plant Physiol. 1977 Apr;59(4):673–679. doi: 10.1104/pp.59.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. K., Lochner N. R. Quantitative estimates of the distribution of homoserine dehydrogenase isozymes in maize tissues. Plant Physiol. 1981 Dec;68(6):1400–1405. doi: 10.1104/pp.68.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA P., GEST H., SEGAL H. L. EFFECTS OF FEEDBACK MODIFIERS ON THE STATE OF AGGREGATION OF HOMOSERINE DEHYDROGENASE OF RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1964 Jan;51:125–130. doi: 10.1073/pnas.51.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P., Epstein C. C. Homoserine dehydrogenase of Rhodospirillum rubrum. Enzyme polymerization in the presence and absence of threonine. Biochemistry. 1973 Sep 25;12(20):3888–3892. doi: 10.1021/bi00744a015. [DOI] [PubMed] [Google Scholar]
- Datta P. Homoserine dehydrogenase of Rhodospirillum rubrum. II. Purification and characterization of the enzyme. J Biol Chem. 1970 Nov 10;245(21):5779–5787. [PubMed] [Google Scholar]
- Dicamelli C. A., Bryan J. K. Changes in Enzyme Regulation during Growth of Maize: II. Relationships among Multiple Molecular Forms of Homoserine Dehydrogenase. Plant Physiol. 1975 Jun;55(6):999–1005. doi: 10.1104/pp.55.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicamelli C. A., Bryan J. K. Comparison of sensitive and desensitized forms of maize homoserine dehydrogenase. Plant Physiol. 1980 Feb;65(2):176–183. doi: 10.1104/pp.65.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M. F., Mennie I., Crofts R. M. The amino acid supplementation of barley for the growing pig. 2. Optimal additions of lysine and threonine for growth. Br J Nutr. 1979 Mar;41(2):333–340. doi: 10.1079/bjn19790042. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S., Bryan J. K. Characterization of ligand-induced states of maize homoserine dehydrogenase. Arch Biochem Biophys. 1983 Nov;227(1):210–224. doi: 10.1016/0003-9861(83)90364-8. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S., Bryan J. K. Ligand-induced interconversions of maize homoserine dehydrogenase among different states. Arch Biochem Biophys. 1983 Apr 15;222(2):449–463. doi: 10.1016/0003-9861(83)90544-1. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S., Bryan J. K. Use of monoclonal antibodies for the purification and characterization of the threonine-sensitive isozyme of maize homoserine dehydrogenase. Arch Biochem Biophys. 1986 Apr;246(1):250–262. doi: 10.1016/0003-9861(86)90471-6. [DOI] [PubMed] [Google Scholar]
- Mankovitz R., Segal H. L. Dissociation-produced loss of regulatory control of homoserine dehydrogenase of Rhodospirillum rubrum. II. Some properties of the regulatable and nonregulatable forms. Biochemistry. 1969 Sep;8(9):3765–3767. doi: 10.1021/bi00837a041. [DOI] [PubMed] [Google Scholar]
- Matthews B. F., Gurman A. W., Bryan J. K. Changes in Enzyme Regulation during Growth of Maize: I. Progressive Desensitization of Homoserine Dehydrogenase during Seedling Growth. Plant Physiol. 1975 Jun;55(6):991–998. doi: 10.1104/pp.55.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. F., Widholm J. M. Expression of aspartokinase, dihydrodipicolinic acid synthase and homoserine dehydrogenase during growth of carrot cell suspension cultures on lysine- and threonine-supplemented media. Z Naturforsch C. 1979 Dec;34(12):1177–1185. doi: 10.1515/znc-1979-1216. [DOI] [PubMed] [Google Scholar]
- Mills W. R. Photosynthetic formation of the aspartate family of amino acids in isolated chloroplasts. Plant Physiol. 1980 Jun;65(6):1166–1172. doi: 10.1104/pp.65.6.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. E., Cromwell G. L., Stahly T. S. Tryptophan, threonine, isoleucine and methionine supplementation of a 12% protein, lysine-supplemented, corn-soybean meal diet for growing pigs. J Anim Sci. 1983 May;56(5):1115–1123. doi: 10.2527/jas1983.5651115x. [DOI] [PubMed] [Google Scholar]
- Walter T. J., Connelly J. A., Gengenbach B. G., Wold F. Isolation and characterization of two homoserine dehydrogenases from maize suspension cultures. J Biol Chem. 1979 Feb 25;254(4):1349–1355. [PubMed] [Google Scholar]