Abstract
Background
Patients with Parkinson's disease have a high dislocation rate after total hip arthroplasty (THA). This study describes a case with severe Parkinson's disease who developed rapidly destructive coxarthrosis (RDC) and underwent THA using a dual mobility cup after a levodopa‐carbidopa intestinal gel (LCIG) infusion.
Case presentation
The patient is a 59‐year‐old female with a ten‐year history of Parkinson's disease, which was first treated with oral levodopa. The patient developed RDC of the right hip joint. However, THA was difficult owing to Parkinson's disease and its treatment side effects, such as wearing‐off, dyskinesia, and freezing of the gait, Thus, LCIG was initiated, and improvement in wearing‐off and dyskinesia was observed. Two months after the LCIG therapy, the disease was controlled well. THA was subsequently performed using a dual mobility cup to prevent postoperative dislocation. Postoperatively, LCIG therapy was continuously administered to carefully manage the disease, which was controlled well with no increase in wearing‐off and dyskinesia after surgery. At 1 year after surgery, the walking speed, stride length, and the Harris hip score improved compared to preoperatively. The UPDRS III motor score improved to eight without signs of wearing‐off or dyskinesia. The Hoehn‐Yahr scale was II in the “on” period and remained unchanged 1 year after surgery. The patient could walk without a cane and had satisfactory functional outcomes.
Conclusion
This case proved that LCIG treatment performed preoperatively, followed by THA using a dual mobility cup, and strict management of Parkinson's disease could result in a satisfactory clinical course without recurrence of wearing‐off and dyskinesia. Similar procedures may benefit other patients with Parkinson's disease who have previously been deemed unsuitable for THA.
Keywords: Intestinal levodopa‐carbidopa gel infusion, Parkinson's disease, Rapidly destructive coxarthrosis, Total hip arthroplasty
The patient with a severe Parkinson's disease developed rapidly destructive coxarthrosis of the right hip joint. Levodopa–carbidopa intestinal gel was initiated, and improvement in wearing‐off and dyskinesia was observed. The patient subsequently underwent total hip arthroplasty using a dual mobility cup and had a satisfactory clinical course without recurrence of wearing‐off and dyskinesia.
Introduction
Parkinson's disease is a neurological disorder with motor symptoms such as akinesia or bradykinesia, rest tremor, rigidity, and postural instability, as well as nonmotor symptoms such as sleep disorders, depression, constipation, and orthostatic hypotension. Damage to mesencephalic substantia nigra neurons depletes dopamine in the striatum, causing an imbalance in basal ganglia function, which results in movement disorders. 1 , 2 Conventional treatment involves oral administration of a dopamine precursor (levodopa). 3 , 4 However, about half of the patients experience motor complications, including dyskinesia and wearing‐off, after 5 years of starting levodopa treatment. 5 Levodopa‐carbidopa intestinal gel (LCIG; Duodopa®), which was developed in Sweden in the 1990s and approved in the EU in 2004 and the USA in 2015, is now available in over 40 countries. 6 LCIG infusion enables continuous administration of levodopa and carbidopa through gastrojejunostomy and inhibits wearing‐off and dyskinesia. 7
Total hip arthroplasty (THA) is becoming a more standardized procedure with high satisfactory rate in patients with hip osteoarthritis, osteonecrosis of the femoral head, and rapidly destructive coxarthrosis (RDC). 8 , 9 , 10 However, patients with Parkinson's disease have a high dislocation rate of 8%–37% after THA. 11 , 12 Although the patients suffer from severe hip pain, they do not prefer undergoing surgery; however, their walking capacity can be comprised if they do not receive appropriate surgical treatment.
To prevent dislocation after THA in patients with Parkinson's disease, appropriate drug management by neurologists after surgery is important. 13 Furthermore, undergoing THA using a dual mobility cup, which is considered a dislocation‐resistant implant, has been demonstrated in a previous study. 11 In this study, we performed THA using a dual mobility cup after introducing LCIG in a patient with severe Parkinson's disease who developed RDC. The patient achieved good postoperative functional and better clinical outcomes with tight control of Parkinson's disease using LCIG could be obtained.
Case Report
A 59‐year‐old female patient with a 10‐year history of Parkinson's disease was treated by administering oral levodopa from the onset of the disease. In March 2018, the patient complained of right hip pain and had difficulty walking without using a Lofstrand cane.
Upon physical examination, the patient was measured to be 155 cm tall and 70 kg in weight with a body temperature of 36.0 °C. The range of motion (ROM) of the right hip was extremely limited. The ROM was 80° in flexion, 10° in extension, 30° in abduction, 20° in adduction, 30° in external rotation, and 10° in internal rotation. The patient's right leg was shorter than the left by 4 cm. The Harris hip score (HHS) was 22.
A hematological examination revealed normal white blood cell count, C‐reactive protein, matrix metalloproteinase‐3, and rheumatoid factor levels (Table 1).
TABLE 1.
Laboratory data
| Parameters | At the time of hospitalization | Normal range |
|---|---|---|
| Complete blood counts | ||
| WBC (/μL) | 3800 | 3300–8600 |
| Neutrocyte (%) | 57.7 | 38.3–74.7 |
| Lymphocytes (%) | 30.1 | 21.2–51.0 |
| Monocytes (%) | 9.1 ↑ | 2.7–8.0 |
| Eosinophils (%) | 2.2 | 0.2–8.4 |
| Basophils (%) | 0.9 | 0.2–2.0 |
| RBC (/μL) | 399 × 104 | 410–530 × 104 |
| Hb (g/dL) | 12.3 | 11.6–14.8 |
| Hct (%) | 37.5 | 35.1–44.4 |
| Plt (/μL) | 24.1 × 104 | 14.0–35.0 × 104 |
| Blood chemistry | ||
| Total protein (g/dL) | 6.4 ↓ | 6.7–8.3 |
| Albumin (g/dL) | 4.1 | 4.0–5.0 |
| AST (IU/L) | 11 ↓ | 13.0–33.0 |
| ALT (IU/L) | 4 ↓ | 6.0–30.0 |
| Urinary nitrogen (mg/dL) | 15.3 | 8.0–22.0 |
| Creatinine (mg/dL) | 0.49 ↓ | 0.60–1.10 |
| Serum sodium (mEq/L) | 142 | 138.0–146.0 |
| Serum potassium (mEq/L) | 4.3 | 3.6–4.9 |
| Serum chloride (mEq/L) | 105 | 99.0–109.0 |
| Immunology | ||
| CRP (mg/dL) | 0.09 | 0.0–0.3 |
| RF (IU/mL) | 5 | 0.0–15.0 |
| MMP‐3 (ng/mL) | 42.4 | 36.9–121.0 |
Note: Each arrow showed abnormal indicators.
Abbreviations: ALT, L‐alanine aminotransferase; AST, L‐aspartate aminotransferase; CRP, C‐reactive protein; Hb, hemoglobin; Hct, hematocrit; MMP‐3, matrix metalloproteinase‐3; Plt, platelet; RBC, red blood cell; RF, rheumatoid factor; WBC, white blood cell.
For the Parkinson's disease condition, despite oral medication therapy (levodopa/benserazide, 700 mg/175 mg, selegiline7.5 mg, entacapone 300 mg (100 mg three times daily), ropinirole 8 mg, rotigotine 18 mg, and istradefylline 20 mg; the total Levodopa equivalent daily dose (LEDD): 1405 mg), the patient suffered from troublesome dyskinesia that alternated to severe “off” periods with an unsteady gait resulting in usage of a Lofstrand cane or wheel chair. The unified Parkinson's disease rating scale (UPDRS) part III motor score was 13 (On) and 48 (Off). The Hoehn‐Yahr scale was III in the “on” period and IV in the “off” period.
Radiographs showed deterioration of the right hip and collapse of the right femoral head (Figure 1). Computed tomography revealed a loss of joint space. A needle aspiration of the right hip was performed to eliminate joint infection, yielding 5 mL of clear yellow fluid. Bacterial culture results were negative. Based on these findings, an RDC diagnosis was made and a surgery was recommended. However, the disease was not well controlled and THA seemed difficult. Therefore, the patient was scheduled for LCIG infusion, followed by THA after consulting with the orthopedic surgeon and neurologist.
FIGURE 1.
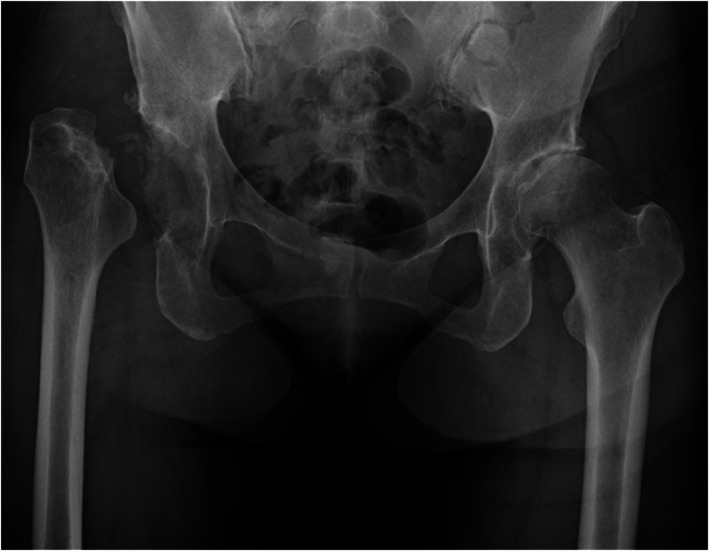
Plain radiograph of the hip joint before surgery. The right femoral head had disappeared and the femur was shortened upward. A narrowing of the joint space was observed in the left hip joint.
In January 2019, percutaneous endoscopic transgastric jejunostomy (PEG‐J) was performed, and levodopa infusion was initiated. After a week of starting the LCIG therapy (morning dose 11.0 ml, continuous dose 3.3 mL/h; 16 h per day, LEDD 1404 mg), motor fluctuations, dyskinesia, gait, and stability were improved. This led to partial recovery in the patient and she was able to perform daily routine activities independently. Psychiatric symptoms were not observed. However, slight dyskinesia was observed in the neck, which did not interfere with her daily life activities. There was pain in the right hip owing to RDC, which remained for 2 months after LCIG therapy.
In April 2019, a right THA combined with subtrochanteric femoral shortening osteotomy was performed using a posterior approach in a lateral decubitus position under general anesthesia. The piriformis muscle was preserved. The G7® Dual mobility cup (multihole, 52 mm diameter; Zimmer‐Biomet Ltd., Warsaw, IN, USA) was placed and fixed using four screws. On the femur side, Modulus R stem® (19 mm diameter; Lima Ltd., Villanova di San Daniele del Friuli, Italy) was inserted. Before stem insertion, a 1‐cm subtrochanteric osteotomy was performed 2 cm below the lesser trochanter due to high soft tissue tension (Figure 2). The short external rotator muscles and the posterior joint capsule were sutured and attached to the greater trochanter using absorbable sutures. Radiographs were taken 1, 3, 6, 9, 12 and 18 months after surgery. Clinical examinations were performed 3, 6, 9 and 12 months after surgery.
FIGURE 2.
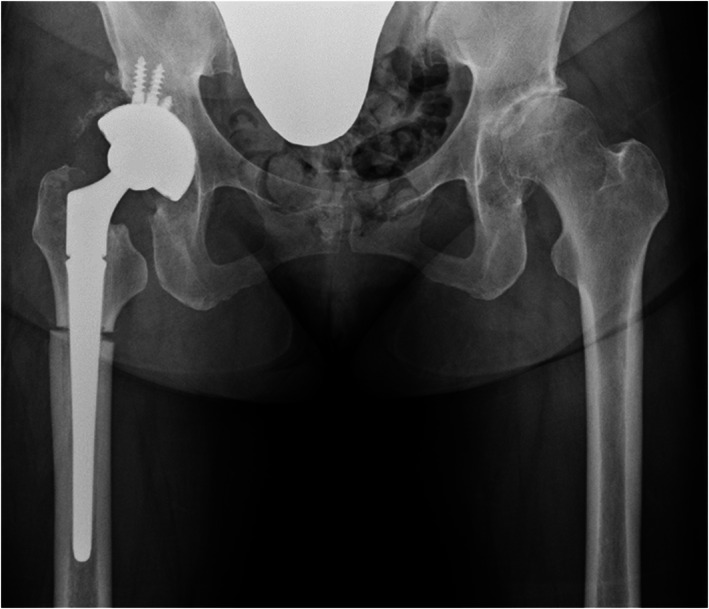
Plain radiograph of the hip joint after surgery. A right THA with subtrochanteric osteotomy was performed.
Regarding postoperative rehabilitation, ROM exercises were advised from the next day of surgery without any restriction. Partial weightbearing was allowed until 2 weeks after the surgery, and full weight bearing was permitted after 8 weeks. Three weeks after the operation, she was transferred to a rehabilitation hospital. Eight weeks after the surgery, she could walk with a cane and was discharged. The ROM was 110° in flexion, 10° in extension, 40° in abduction, 20° in adduction, 30° in external rotation, and 10° in internal rotation during the final follow‐up. The right leg was shorter than the left by 0.2 cm. The HHS improved to 74 points. Complete bone union at the osteotomy site was observed during the follow‐up conducted after a year of surgery (Figure 3). Furthermore, a radiograph showed the radiolucent line around the acetabular component at zone 3 and cortical hypertrophy around the stem at zone 2 at 18 months after operation (Figure 4). The patient had no pain complaints and no postoperative complications, such as subsidence of the stem, hip joint dislocation, or infection were observed.
FIGURE 3.
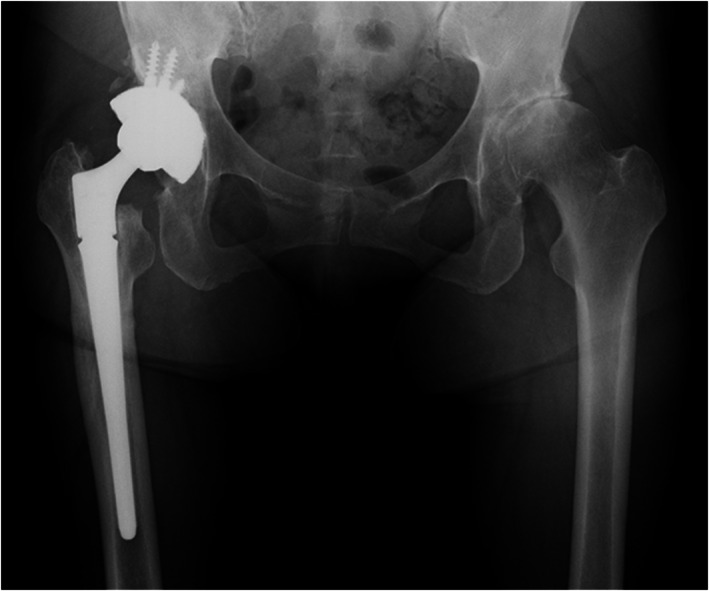
Plain radiograph of the hip joint 1 year after operation. Bone union at the osteotomy site was observed.
FIGURE 4.
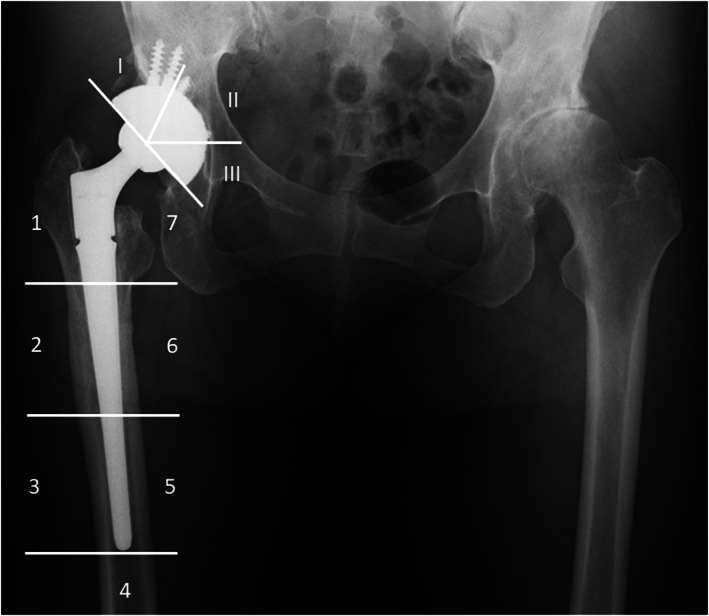
Plain radiograph of the hip joint showing the seven femoral zones and the three acetabular zones 18 months after operation. Radiolucent line in zone 3 and cortical hypertrophy in zone 2 were observed with no stem subsidence.
Regarding the Parkinson's disease condition after surgery, the UPDRS III motor score improved to eight (On) without signs of wearing‐off or troublesome dyskinesia and remained unchanged until the final follow‐up. The Hoehn‐Yahr scale was II in the “on” period and did not change pre‐ and postoperatively. The clinical results revealed no changes in the range of motion of the hip joint and lower extremity muscle strength pre‐ and postoperatively. Gait speed and the HHS tended to increase until 12 months postoperatively (Table 2).
TABLE 2.
Clinical outcomes in follow‐up duration
| At the time of surgery | Post‐op 3 months | Post‐op 6 months | Post‐op 12 months | |
|---|---|---|---|---|
| ROM (degrees) | ||||
| Flexion | 80 | 110 | 110 | 110 |
| Extension | 10 | 10 | 10 | 10 |
| Abduction | 30 | 40 | 40 | 40 |
| Adduction | 20 | 20 | 20 | 20 |
| External rotation | 30 | 30 | 30 | 30 |
| Internal rotation | 10 | 10 | 10 | 20 |
| MMT | ||||
| Iliopsoas | 3 | 4 | 4 | 4 |
| Gluteus medius | 3 | 3 | 4 | 4 |
| Quadriceps femoris | 5 | 5 | 5 | 5 |
| Tibialis anterior | 5 | 5 | 5 | 5 |
| Gait analysis | ||||
| Gait speed (cm/s) | 23.1 | N/R | 29.2 | 37.6 |
| Stride length (Rt/Lt, cm) | 32/30 | N/R | 56/55 | 49/51 |
| Harris hip score | 22 | 51 | 63 | 74 |
| UPDRS part III motor score | 10 | 8 | 5 | 8 |
| Hoehn‐Yahr scale | II | II | II | II |
Abbreviations: MMT, manual muscle testing; N/R, not recorded; ROM: range of motion.
Discussion
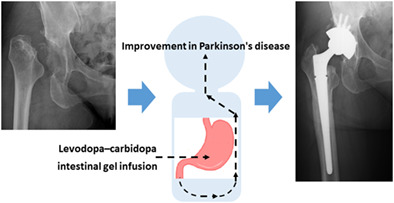
We studied RDC in a patient with LCIG introduction to control severe Parkinson's disease symptoms. After introducing LCIG, THA was performed, resulting in less pain complaints and improvement in the walking ability. LCIG (Duodopa®) is a method for injecting a gel‐like combination of levodopa and carbidopa continuously into the jejunum using a portable pump through an indwelling tube from gastrojejunostomy. 7 Wearing‐off and dyskinesia are suppressed by avoiding fluctuations in levels of levodopa in blood through continuous infusion of levodopa. 14 As abrupt discontinuation of oral levodopa causes changes in Parkinson's symptoms, we generally switch to a levodopa infusion when oral administration is difficult during the pre‐ and postoperative periods. However, because there is no established index for conversion, the optimal dosage differs between oral preparations and dopamine injection. Thus, perioperative Parkinson's disease seems difficult to control. However, LCIG administration through gastrojejunostomy can be continued, which might suppress the fluctuations in the symptoms. In patients with severe Parkinson's disease, similar to this case, using LCIG induces mild perioperative dyskinesia with no “off” symptoms. Patients with Parkinson's disease experience perioperative complications, such as pneumonia if their conditions are poorly managed. 13 , 15 Therefore, perioperative management using LCIG for patients with Parkinson's disease could be useful in preventing disease conditions and perioperative complications. Postural instability occurs at all stages of Parkinson's disease and becomes increasingly common with disease progression. Clinical observations indicate that postural instability can be successfully treated with dopaminergic therapy in early to mid‐stage Parkinson's disease. However, postural instability has been less responsive to dopaminergic therapy than other major symptoms of Parkinson's disease. 16 Deep brain stimulation and LCIG for advanced Parkinson's disease are effective against motor fluctuations and dyskinesias. However, the effects of these treatments on postural instability have not yet been fully elucidated. 17 Thus, postural instability with falls and risk for dislocation remains a problem in patients with Parkinson's disease despite advanced therapies. A schematic illustration shows a procedure in management of Parkinson's disease patients with hip disorders (Figure 5).
FIGURE 5.
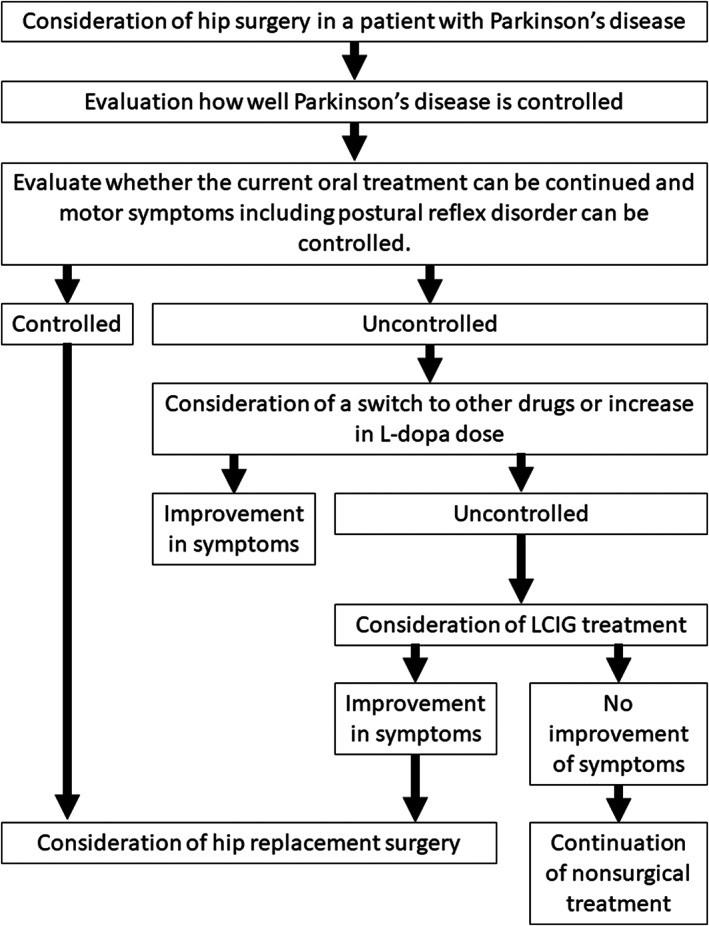
A schematic illustration shows a procedure in the management of patients with Parkinson's disease with hip disorders.
Postoperative complications such as dislocation, periprosthetic fracture, aseptic loosening, and surgical site infection have been reported in patients with Parkinson's disease undergoing THA (Table 3). 18 , 19 , 20 , 21 , 22 , 23 , 24 Among them, THA in patients with Parkinson's disease has a high risk of postoperative dislocation. In a study in the 1980s, it has been reported that a dislocation rate after THA is 37%. 19 However, recent studies indicate better dislocation outcome rates of 2%–10%. 11 , 12 , 20 , 22 , 23 , 24 Falls due to muscle weakness or postural disturbance are the main reason for dislocation. 11 Additionally, periprosthetic fractures have been reported in 1%–10% of patients. 11 , 12 , 21 , 22 , 23 , 24 Thus, drug therapy aimed at maintaining muscle tone is considered important to prevent dislocations and periprosthetic fractures associated with falls. Conversely, surgical site infections have been reported in up to 22% of patients postoperatively. 11 , 12 , 20 , 22 , 24 Furthermore, urinary tract infections and pneumonia occur in the early postoperative period. 13 Therefore, the use of antibiotics should be considered to prevent these infections in the early postoperative period. This study revealed no falling, postoperative surgical site infections, or other infections during the follow‐up period. Weber et al. examined THA outcomes in 107 joints in 98 patients with Parkinson's disease with a mean follow‐up of 7.1 years. Functional outcomes improved early in follow‐up but worsened as the neurological status of Parkinson's disease worsened. The Hoehn‐Yahr scale at the time of surgery was I for 14 patients, II for 52 patients, III for 38 patients, IV for two patients, and unknown for one patient. At the final follow‐up, 57% of patients had reportedly progressed to Hoehn‐Yahr scale IV or V. Additionally, postoperative complications were observed at a high rate of 26%, reflecting the increased incidence of complications associated with Parkinson's disease. 24 This study revealed the Hoehn‐Yahr scale of III in the “on” period before LCIG treatment but improved to II after LCIG treatment. The Hoehn‐Yahr scale after THA was maintained during the follow‐up periods with continued LCIG treatment. Therefore, cooperating with neurologists in the management of patients with Parkinson's disease seems important to prevent neurological disorder progression from the viewpoint of decreasing postoperative complications. A schematic illustration showing the procedure in patient management is summarized in Figure 5.
TABLE 3.
Study, revision rate, and surgical complications in patients with Parkinson's disease undergoing THA
| Study | Number | Mean age (years) | Average follow‐up period (years) | Revision rate | Surgical complications |
|---|---|---|---|---|---|
| Shah et al .20 | 235 | 74.3 | 2 | 2‐year (6.4%) | Surgical site infections (11.1%); Dislocation (3.0%) |
| Rong et al. 21 | 24 | 64.7 | 4.3 | 5‐year (5.9%) | Intraoperative fracture (3.6%) |
| Wojtowicz et al. 22 | 490 | 73 | 13 |
90‐day (1.0%) 1‐year (2.1%) 9‐year (5.4%) |
Surgical site infections (22%); Aseptic loosening (10%); Periprosthetic fracture (4%); Dislocation (8%) |
| Lazennec et al. 11 | 59 | 72.5 | 8.3 |
2‐year (8.5%) 5‐year (20.3%) |
Surgical site infections (3.3%); Periprosthetic fracture (6.8%); Dislocation (1.7%) |
| Rondon et al.12 | 52 | 68.7 | 5.3 |
2‐year (5.7%) 5‐year (14.7%) 10‐year (21.3%) |
Surgical site infections (1.9%); Aseptic loosening (7.7%); Periprosthetic fracture (7.7%); Dislocation (7.7%) |
| Šponer et al. 23 | 10 | 74 | 6.8 | N/R | Periprosthetic fracture (10%); Dislocation (10%) |
| Weber et al.24 | 107 | 72 | 7.1 | 5‐year (7%) | Surgical site infections (0.9%); Aseptic loosening (2.8%); Periprosthetic fracture (0.9%); Dislocation (5.6%) |
Abbreviation: N/R, Not recorded.
Generally, a dual mobility cup, large‐diameter femoral head, and constraining liner have been used to prevent postoperative dislocation in THA. Among these, the dual mobility cup is the most effective in preventing dislocation. 25 Caton et al. followed up on the initial THA in 320 joints for more than ten years and reported that the postoperative dislocation rate was significantly lower in the patients using the dual mobility cup (1/105 joint; 0.9%) compared to the normal cup (26/215 joint; 12.9%). 26 Lazennec et al. reported that postoperative dislocation was observed in only one joint in 59 THA joints of patients with Parkinson's disease using a dual mobility cup 9 years after surgery. 11 The patient in this study showed improvement in walking ability without postoperative dislocation. Therefore, THA with a dual mobility cup could be a valuable treatment option for patients with Parkinson's disease.
Systematic review describing surgical approaches for dislocation rates after THA showed no significant difference in the dislocation rates between the anterior, lateral, and posterior approaches with soft tissue repair in patients without neurological disorders (anterior: 0.70%; lateral: 0.43%; and posterior: 1.01%). 27 To the best of our knowledge, there are no studies reporting differences between dislocation rates and surgical approaches for THA in patients with Parkinson's disease. In this study, the right lower limb was shortened by 4 cm compared to the left lower limb due to the loss of the femoral head by RDC. Even with femoral shortening osteotomy, additional soft tissue release may be required if it is tight during femoral head reduction. Therefore, the posterior approach was used, allowing for a wider operative field. Furthermore, we surgically repaired the posterior hip external rotators, and the patient showed no dislocation after surgery. Thus, a posterior approach with a dual mobility cup and posterior repair is considered safe. As there is a risk of dislocation even with THA using a dual mobility cup, performing rehabilitation and improving the living conditions after surgery are necessary.
The retraction of the femur becomes challenging after a long period following the onset of RDC, which may also cause neuropathy after surgery. In this study, we planned to pull down the femur by 3 cm preoperatively. Notably, leg lengthening of more than 8.7% of femoral length causes neuropathy in 66% of patients in THA. 28 This patient had a femur length of 40 cm and we determined that leg lengthening should be up to 3.5 cm without subtrochanteric osteotomy. Intraoperative findings showed strong tension in the hip joint. Therefore, we performed a 1‐cm subtrochanteric osteotomy and did not observe postoperative neuropathy.
Limitations
This study has several limitations and little information on the long‐term efficacy and safety of LCIG is available due to its novelty. The follow‐up time was short. Postoperative radiographs showed a radiolucent line around the acetabular component and cortical hypertrophy around the stem, which indicated requirement of radiographic follow‐up.
Conclusion
In conclusion, THA was performed after LCIG treatment for RDC in a patient with Parkinson's disease prior surgery. Performing THA using a dual mobility cup, and strict management of Parkinson's disease induced a favorable clinical course without recurrence of wearing‐off or dyskinesia.
Author Contributions
Atsushi Imamura conceptualized, wrote, and edited the manuscript together with Gen Kuroyanagi, Takuya Usami, Toyohiro Sato, Mitsuya Horiba, Hiroaki Sakai, Ayaka Takahashi, Yoshino Ueki, Noriyuki Matsukawa, Hideki Murakami. All authors read and approved the manuscript.
Conflict of Interest Statement
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This investigation was supported in part by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant number 19K18471).
References
- 1. Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet. 2021;397(10291):2284–2303. [DOI] [PubMed] [Google Scholar]
- 2. Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50(2):140–148. [DOI] [PubMed] [Google Scholar]
- 3. Pringsheim T, Day GS, Smith DB, Rae‐Grant A, Licking N, Armstrong MJ, et al. Dopaminergic therapy for motor symptoms in early Parkinson disease practice guideline summary: a report of the AAN guideline subcommittee. Neurology. 2021;97(20):942–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deuschl G, Antonini A, Costa J, Śmiłowska K, Berg D, Corvol JC, et al. European academy of neurology/Movement Disorder Society‐European section guideline on the treatment of Parkinson's disease: I. Invasive Therapies Eur J Neurol. 2022;29(9):2580–2595. [DOI] [PubMed] [Google Scholar]
- 5. Ahlskog JE, Muenter MD. Frequency of levodopa‐related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448–458. [DOI] [PubMed] [Google Scholar]
- 6. Wirdefeldt K, Odin P, Nyholm D. Levodopa‐carbidopa intestinal gel in patients with Parkinson's disease: a systematic review. CNS Drugs. 2016;30(5):381–404. [DOI] [PubMed] [Google Scholar]
- 7. Tsunemi T, Oyama G, Saiki S, Hatano T, Fukae J, Shimo Y, et al. Intrajejunal infusion of levodopa/carbidopa for advanced Parkinson's disease: a systematic review. Mov Disord. 2021;36(8):1759–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuroda Y, Okuzu Y, Kawai T, Goto K, Matsuda S. Difference in therapeutic strategies for joint‐preserving surgery for non‐traumatic osteonecrosis of the femoral head between the United States and Japan: a review of the literature. Orthop Surg. 2021;13(3):742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baryeh K, Asopa V, Field R, Sochart DH. The outcomes of total hip arthroplasty in rapidly progressive osteoarthritis: a systematic review. Eur J Orthop Surg Traumatol. 2022;33:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martina K, Hunter DJ, Salmon LJ, Roe JP, Dowsey MM. Surgery for osteoarthritis: Total joint arthroplasty, realistic expectations of rehabilitation and surgical outcomes: a narrative review. Clin Geriatr Med. 2022;38(2):385–396. [DOI] [PubMed] [Google Scholar]
- 11. Lazennec JY, Kim Y, Pour AE. Total hip arthroplasty in patients with Parkinson disease: improved outcomes with dual mobility implants and Cementless fixation. J Arthroplasty. 2018;33(5):1455–1461. [DOI] [PubMed] [Google Scholar]
- 12. Rondon AJ, Tan TL, Schlitt PK, Greenky MR, Phillips JL, Purtill JJ. Total joint arthroplasty in patients with Parkinson's disease: survivorship, outcomes, and reasons for failure. J Arthroplasty. 2018;33(4):1028–1032. [DOI] [PubMed] [Google Scholar]
- 13. Queally JM, Abdulkarim A, Mulhall KJ. Total hip replacement in patients with neurological conditions. J Bone Joint Surg Br. 2009;91(10):1267–1273. [DOI] [PubMed] [Google Scholar]
- 14. Nyholm D, Odin P, Johansson A, Chatamra K, Locke C, Dutta S, et al. Pharmacokinetics of levodopa, carbidopa, and 3‐O‐methyldopa following 16‐hour jejunal infusion of levodopa‐carbidopa intestinal gel in advanced Parkinson's disease patients. AAPS J. 2013;15(2):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pepper PV, Goldstein MK. Postoperative complications in Parkinson's disease. J Am Geriatr Soc. 1999;47(8):967–972. [DOI] [PubMed] [Google Scholar]
- 16. Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson's disease and their response to treatment. Br J Clin Pharmacol. 2012;74(2):267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim SD, Allen NE, Canning CG, Fung VS. Postural instability in patients with Parkinson's disease. Epidemiology, pathophysiology and management. CNS Drugs. 2013;27(2):97–112. [DOI] [PubMed] [Google Scholar]
- 18. Fontalis A, Kenanidis E, Bennett‐Brown K, Tsiridis E. Clinical outcomes in elective total hip arthroplasty in Parkinson's disease: a systematic review of the literature. EFORT Open Rev. 2020;5(12):856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coughlin L, Templeton J. Hip fractures in patients with Parkinson's disease. Clin Orthop Relat Res. 1980;148:192–195. [PubMed] [Google Scholar]
- 20. Shah NV, Solow M, Lavian JD, Bloom LR, Grieco PW, Stroud SG, et al. Total hip arthroplasty in Parkinson's disease patients: a propensity score‐matched analysis with minimum 2‐year surveillance. Hip Int. 2020;30(6):684–689. [DOI] [PubMed] [Google Scholar]
- 21. Rong X, Dahal S, Luo ZY, Zhou K, Yao SY, Zhou ZK. Functional outcomes after total joint arthroplasty are related to the severity of Parkinson's disease: a mid‐term follow‐up. J Orthop Surg Res. 2019;14(1):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wojtowicz AL, Mohaddes M, Odin D, Bülow E, Nemes S, Cnudde P. Is Parkinson's disease associated with increased mortality, poorer outcomes scores, and revision risk after THA? Findings from the Swedish hip arthroplasty register. Clin Orthop Relat Res. 2019;477(6):1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Šponer P, Kučera T, Grinac M, Bezrouk A, Waciakowski D. The outcomes of Total hip replacement in patients with Parkinson's disease: comparison of the elective and hip fracture groups. Parkinsons Dis. 2017;2017:1597463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber M, Cabanela ME, Sim FH, Frassica FJ, Harmsen WS. Total hip replacement in patients with 185 Parkinson's disease. Int Orthop. 2002;26(2):66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pituckanotai K, Arirachakaran A, Tuchinda H, Putananon C, Nualsalee N, Setrkraising K, et al. Risk of revision and dislocation in single, dual mobility and large femoral head total hip arthroplasty: systematic review and network meta‐analysis. Eur J Orthop Surg Traumatol. 2018;28(3):445–455. [DOI] [PubMed] [Google Scholar]
- 26. Caton JH, Prudhon JL, Ferreira A, Aslanian T, Verdier R. A comparative and retrospective study of three hundred and twenty primary Charnley type hip replacements with a minimum follow up of ten years to assess whether a dual mobility cup has a decreased dislocation risk. Int Orthop. 2014;38(6):1125–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop Relat Res. 2006;447:34–38. [DOI] [PubMed] [Google Scholar]
- 28. Kabata T, Kajino Y, Inoue D, Ohmori T, Yoshitani J, Ueno T, et al. Safety range for acute limb lengthening in primary total hip arthroplasty. Int Orthop. 2019;43(9):2047–2056. [DOI] [PubMed] [Google Scholar]


