ABSTRACT.
Low-income countries carry approximately 90% of the global burden of visual impairment, and up to 80% of this could be prevented or cured. However, there are only a few studies on the prevalence of retinal disease in these countries. Easier access to retinal information would allow differential diagnosis and promote strategies to improve eye health, which are currently scarce. This pilot study aims to evaluate the functionality and usability of a tele-retinography system for the detection of retinal pathology, based on a low-cost portable retinal scanner, manufactured with 3D printing and controlled by a mobile phone with an application designed ad hoc. The study was conducted at the Manhiça Rural Hospital in Mozambique. General practitioners, with no specific knowledge of ophthalmology or previous use of retinography, performed digital retinographies on 104 hospitalized patients. The retinographies were acquired in video format, uploaded to a web platform, and reviewed centrally by two ophthalmologists, analyzing the image quality and the presence of retinal lesions. In our sample there was a high proportion of exudates and hemorrhages—8% and 4%, respectively. In addition, the presence of lesions was studied in patients with known underlying risk factors for retinal disease, such as HIV, diabetes, and/or hypertension. Our tele-retinography system based on a smartphone coupled with a simple and low-cost 3D printed device is easy to use by healthcare personnel without specialized ophthalmological knowledge and could be applied for the screening and initial diagnosis of retinal pathology.
INTRODUCTION
It is estimated that 285 million people are moderate to severely sight impaired worldwide, of which 43 million are blind.1 Low- and middle-income countries (LMICs) carry approximately 90% of the global burden of visual impairment, and it has been estimated that up to 80% of this impairment could be prevented or cured.2 The WHO highlights the lack of robust survey data as a key challenge to deal with and manage eye and visual impairment, particularly in some countries of sub-Saharan Africa (SSA).3
In this context, universal and periodic screening could drastically reduce the risk of these diseases by detecting early manifestations, thus fostering prompt preventive and therapeutic interventions. Fundus visualization is a fundamental and noninvasive tool in the diagnosis and treatment of eye diseases such as diabetic retinopathy or age-related macular degeneration.4,5 Furthermore, this is a key element in the diagnosis of other systemic, neurologic, or cerebrovascular diseases.6,7 It also has high potential in the diagnosis of diseases such as Alzheimer’s8 and for the early detection of severe stages of diseases for which a characteristic sign includes retinal lesions, such as cerebral malaria9,10 or cytomegaloviral disease.11,12 Affordable diagnostic equipment, local training, and development of systems for early detection and treatment of several retinal diseases could help the diagnosis of visual impairment and retinal disease in SSA.13
There are several recent designs of retinographs developed with different strategies. The first are portable retinographs that are lighter and easier to transport, such as Smartscope Pro (Optomed, Oulu, Finland), Epicam M (Epipole, Dunfermline, United Kingdom), VersaCamTM DS-10 (Nidek, Gamagori, Japan), or Horus DEC 200 (MiiS, Hsinchu, Taiwan).14 More prototypes are under development including by Epipole and IDx (Iowa City, IA).15 On the other hand, the second line that is being developed uses Smartphones for image acquisition and processing such as Peek Vision (Nesta, London, United Kingdom),16 PanOptic iExaminer (Welch Allyn, Skaneateles Falls, NY), and D-Eye (D-EYE, Padova, Italy).17 Some of these solutions are mydriatic—that is, they require the patient to receive eye drops for pupil dilation (e.g., Peek Vision and D-Eye). Another typical characteristic is limited fields of view (25° and 20°, respectively, for PanOptic and D-Eye). Other specific fundus cameras that include a larger and more sophisticated adapter are also available in the market, including, for instance, the Remidio FOP-NM (Bangalore, India)18; Volk iNview and Vistaview (Volk Optical, Mentor, OH)19; Cellscope Retina, which is 3D-printed (University of Michigan, MI)20; MII Retcam (Coimbatore, India)21; and Phelcom Eyer (São Carlos, Brazil).22 A comprehensive price review of the previous 15 portable fundus cameras was conducted, with a median price of ∼$3,000. These were classified into three price groups according to their market price regarding multiple sources: one was more than $10,000, six were between $1,000 and $10,000 and three were less than $1,000.
Easy-to-use, retinal-screening devices that could be deployed in settings without current access to complex and expensive technology will help fill existing screening and diagnostic gaps.23 Rates of screening for ocular diseases could be increased with the availability of portable, low-cost, easy-to-use medical devices, which could enable capturing retinal images and/or videos, allowing their interpretation by specialists on-site or through distance tele-diagnosis methods. In this respect, the combination of 3D printing, smartphones, and remote connection systems could be a potential new approach for visualizing the fundus of the eye.
We present here the design of a low-cost portable retinal scanner manufactured with 3D printing and controlled by a smartphone connected to a tele-retinography web platform, as well as the results of a pilot study to evaluate its utility to screen fundus pathology in an adult population in Manhiça, southern Mozambique.
MATERIALS AND METHODS
Study design and ethical considerations.
Because this was a pilot study, we calculated an opportunistic sample size of 100 patients, with no predefined sample size calculations based on expected prevalence of retinal disease. The study recruitment took place between October 2017 and January 2018. Each morning, the study staff randomly selected patients from all the new adult admissions in Manhiça District Hospital (MDH). Patients attending the MDH who met the inclusion criteria were offered enrollment into the study. Exclusion criteria included patients with a low level of consciousness, critically ill patients who could not collaborate, and/or refusal to participate in the study or sign the informed consent. A simple, standardized, study-specific questionnaire was completed upon recruitment. Demographic and basic clinical data were recorded. Information on the presence of other pathologies such as HIV, hypertension, and/or diabetes was collected from all recruited patients.
Study setting and participants.
In Mozambique, there were an estimated 2.7 million people with vision loss in 2020. Of those, 110,000 people were blind.1 For those with severe visual impairment, untreated cataract was the main cause with 71.2%, followed by other posterior segment disease and uncorrected refractive error (6.8% and 5.91%, respectively). For moderate visual impairment, untreated cataract and uncorrected refractive errors (46.5% and 40.8%, respectively) were the main underlying factors.24 This pilot study was conducted by CISM at MDH, in southern Mozambique. A detailed description of CISM may be found elsewhere.25 MDH is one of two public referral hospitals in the district, covering a total population of ∼205,000 people. Approximately 900 to 1,000 adult patients are admitted each year in the adult wards. Ophthalmology services for the population are provided at MDH by an ophthalmology technician who runs an outpatient clinic. No retinal screening devices are available in the hospital, and patients suspected to have retinal pathology require referral to Maputo’s central hospital, where the only fully trained Mozambican ophthalmologist in the country can provide clinical assistance.
Mobile device for digitalization of fundus eye and telemedicine platform.
In our study, acquisition of fundus videos was performed using an original, low-cost portable device composed of a 3D-printed plastic structure and a lens that uses a smartphone app that converts a smartphone into a retinal-screening device. Videos from the retina are uploaded to a platform that allowed two independent ophthalmologists to review the cases.
Hardware 3D-printed plastic structure.
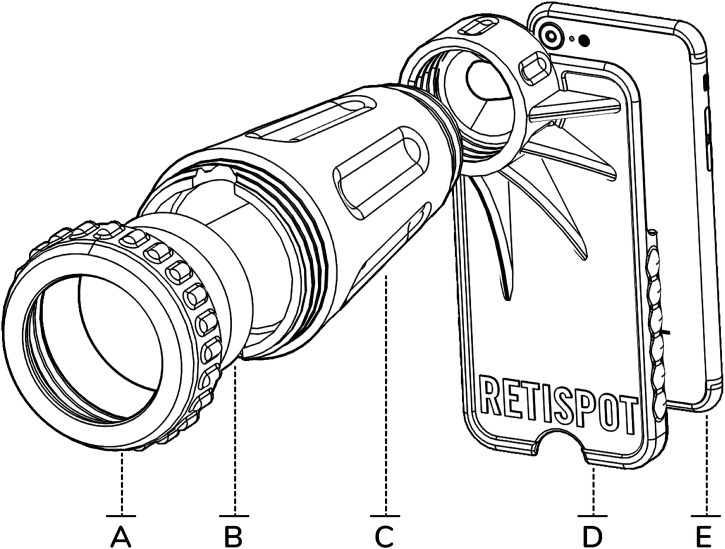
To visualize and digitize the retina, three components are required: a light source, an ophthalmic lens, and a camera. The device uses the flash of the smartphone camera as a light source, which is aligned with the camera sensor. The smartphone used in this study was an iPhone 6 with the Volk Pan Retinal 2.2 lens (296.80 €/unit). To fix the alignment of these three components, a handheld and detachable structure made with 3D printing was used. The printing of the device was done with the Hephestos 2 printer from BQ (Madrid, Spain) and with 136 gr polylactic acid filament of 1.75 mm (2.76€) used from the same company. The arrangement of the different elements and the light beams are depicted in Figure 1. Computer-aided design is open for research use and available upon request.
Figure 1.
Hardware of 3D-printed plastic structure. (A) Cover for attaching the lens to the structure. (B) Ophthalmic lens (Pan Retinal Lens, Volk 2.2). (C) Cone for ophthalmic lens attachment and external light isolation. (D) Case for attachment of the mobile phone. (E) Smartphone.
Smartphone app.
Viewing and recording of videos was possible via a smartphone application designed for this purpose. Access to this application was protected by personal authentication. The mobile app used in this study has been specifically designed for retinal screening purposes. To increase performance, some special features have been included to improve usability:
Videos were acquired instead of taking still images; frames in which the retina is best visualized could then be chosen and extracted semiautomatically.
Images underwent automated inversion to help with the acquisition of videos and their interpretation. This feature is of great help for the clinician using the device or the nonclinical operator who needs to capture the image, given that the Volk lens inverts the image and makes the traditional use of retinography counterintuitive. In the app, this inversion was corrected, and the interpretation of the images corresponds to a more intuitive movement.
The user could adjust the intensity of the flash to suit the patient.
The videos were acquired without audio to reduce their file size.
Data were uploading into the cloud to the telemedicine platform using automation.
Auto-focus routines within the smartphone camera module were used to correct refractive focusing defects such as myopia or hyperopia.
Telemedicine review platform.
We created a customized telemedicine platform, built on Amazon Web Services, that allowed online storage, user access control, remote visualization of videos organized by cases, and annotation of relevant findings. The smartphone app synchronizes its local database with the one backed up in the cloud by downloading configuration updates on the acquisition protocols (such as patient form templates) and by uploading the acquired images along with, and in the context of, the patient metadata that would be gathered from the patient form templates.
Acquisition and analyses of fundus videos.
To observe the retina properly, it was necessary to dilate the pupils using mydriatic eye drops. Mydriasis was achieved by instilling drops of tropicamide (two drops in each pupil), diluted to 1%. After instillation, a minimum of 30 minutes was allowed to elapse for the drug to act completely on the individual’s pupils. Patients were observed in a quiet and semidark environment. Patients with insufficient pupil dilation, cataract, or any other media opacity that prevented a clear view of the fundus were excluded.
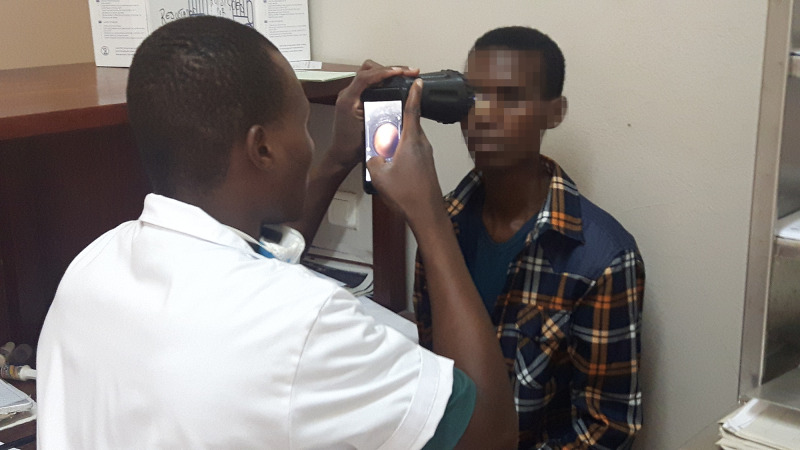
For each patient, two fundus videos per eye were acquired with the device. The study personnel (two general practitioners with no specific knowledge of ophthalmology and no experience using an ophthalmoscope) were responsible for acquiring the images (Figure 2). A 3-hour practical training was given before the onset of patient recruitment.
Figure 2.
General practitioner acquiring a video of the retina with the retinal screening device.
The videos recorded were stored securely and presented in an easy-to-use dashboard that allows their visualization for specialized evaluation via a telemedicine platform by two ophthalmologists from the Institut Català de Retina in Barcelona, Spain. To perform a blinded evaluation of the videos, the collected information from each patient was not provided.
Both experts viewed the videos independently and noted the following for each eye: 1) the quality of the videos (classified as good, sufficient, and poor); 2) the presence of any of 10 fundus alterations including drusen, cotton wool spots or hard exudates, hemorrhages, retinal atrophy, neuroretinal rim thinning, neovascularization, pathological artery-to-vein ratio, retinal whitening, whitening of retinal vessels, and others; 3) “probable pathology” (patients whose retinal lesions were compatible with retinal or optic nerve pathology but needed confirmatory test), and 4) comments.
Discrepant findings were discussed and resolved jointly by both experts. Results of this assessment were sent back to the study team in Manhiça within 24 hours. In case of pathological findings, a detailed report including the images captured were prepared, so that adequate follow-up procedures could be organized through the outpatient ophthalmology consultations at MDH or, if required, through the ophthalmology department at the referral quaternary hospital in Maputo. In addition, technical data of the device (duration and quality of videos and visualization of the optic nerve and the macula) were recorded to assess usability and measure the handling ease of the device.
RESULTS
Baseline characteristics of patients.
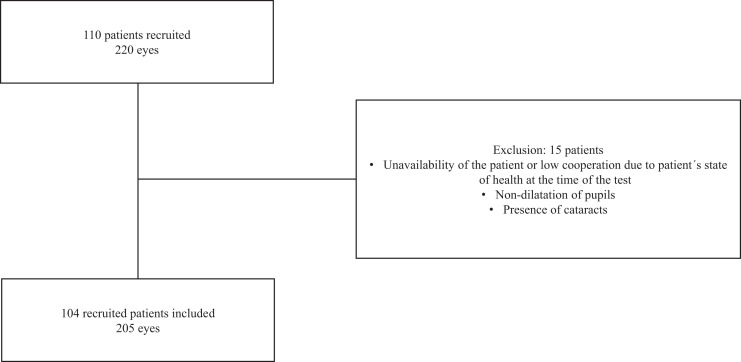
A total of 110 adult patients were recruited at MDH, Mozambique, of whom six were excluded from the study because of cataracts, insufficient patient cooperation, or insufficient pupil dilation. Therefore, data from 104 patients and 205 eyes were analyzed (Figure 3).
Figure 3.
Flow chart followed for the inclusion of patients in the study.
Patients’ ages ranged between 18 and 82 years old (median age: 41 ± 13); 48% were men, and 52% were women (Table 1). The most common pathologies for admission were pneumonia, anemia, and gastroenteritis. It should be noted that 56.7% (59/104) of the patients included in the study were known to be HIV-positive, and 13.6% (8/59) of the HIV patients were not taking antiretroviral therapy. In addition, 3.9% and 15.4% of patients had a known history of diabetes and systemic arterial hypertension, respectively. Information on ocular symptoms history was obtained from a patient questionnaire. Of the total population, 42.3% of the patients complained of visual symptoms; 33.7% presented blurred vision, 18.3% ocular pain, 16.4% loss of vision, 15.4% sensation of shadows in the eyes, and 1.9% sensation of double vision.
Table 1.
Descriptive data of the study participants, ocular findings and relationship between previous pathologies and ocular lesions
| Descriptive data | General | Ocular lesions | |||||
|---|---|---|---|---|---|---|---|
| Exudate | Hemorrhage | Neuroretinal rim thinning | Pathological artery–vein | Other | Any lesion | ||
| N | % | % | % | % | % | % | |
| Sex | |||||||
| Men | 49 | 16.33 | 8.16 | 4.08 | 8.16 | 8.16 | 30.61 |
| Women | 55 | 3.64 | 1.82 | 1.82 | 3.64 | 0.00 | 7.27 |
| Previous pathology | |||||||
| No known diabetes, hypertension, HIV | 35 | 8.57 | 2.86 | 2.86 | 2.86 | 0.00 | 11.43 |
| HIV | |||||||
| Yes | 59 | 8.47 | 3.39 | 3.39 | 6.78 | 5.08 | 18.64 |
| Taking antiretrovirals | 51 | 5.88 | 3.92 | 1.96 | 7.84 | 3.92 | 15.69 |
| Not taking antiretrovirals | 8 | 25.00 | 0.00 | 12.50 | 0.00 | 12.50 | 37.50 |
| No | 27 | 3.70 | 3.70 | 0.00 | 7.41 | 0.00 | 11.11 |
| Unknown | 18 | 22.22 | 11.11 | 5.56 | 0.00 | 5.56 | 27.78 |
| Diabetes | |||||||
| Yes | 4 | 25.00 | 25.00 | 0.00 | 0.00 | 0.00 | 25.00 |
| No | 99 | 9.09 | 4.04 | 3.03 | 6.06 | 4.04 | 18.18 |
| Unknown | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hypertension | |||||||
| Yes | 16 | 12.50 | 6.25 | 0.00 | 12.50 | 12.50 | 31.25 |
| Taking antihypertensives | 11 | 18.18 | 9.09 | 0.00 | 18.18 | 9.09 | 36.36 |
| Not taking antihypertensives | 5 | 0.00 | 0.00 | 0.00 | 0.00 | 20.00 | 20.00 |
| No | 87 | 9.20 | 4.60 | 3.45 | 4.60 | 2.30 | 16.09 |
| Unknown | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 104 | 19.96 | 10 | 6 | 12 | 8 | 38 |
Video acquisition.
Two videos for each eye were recorded in each patient, and a total of 410 videos from 205 eyes were obtained. In each eye, an attempt was made to digitize the optic nerve and the macula. In 98% of the videos, the optic nerve was visible. Both the macula and optic nerve were seen in 88% of the videos. The mean duration of the videos was 17 ± 5 seconds (range 4–29 seconds). Regarding the video quality, in 56% (n = 114) of the videos, the experts agreed that the quality was good; in 30% (n = 62), it was sufficient; and in 7% (n = 15), the quality was poor. Discrepancies were observed in 5% (n = 10) of the videos where one of the experts considered the quality to be good and the other sufficient, and in 2% (n = 4), one of the experts considered the quality to be sufficient and the other poor.
Fundus findings.
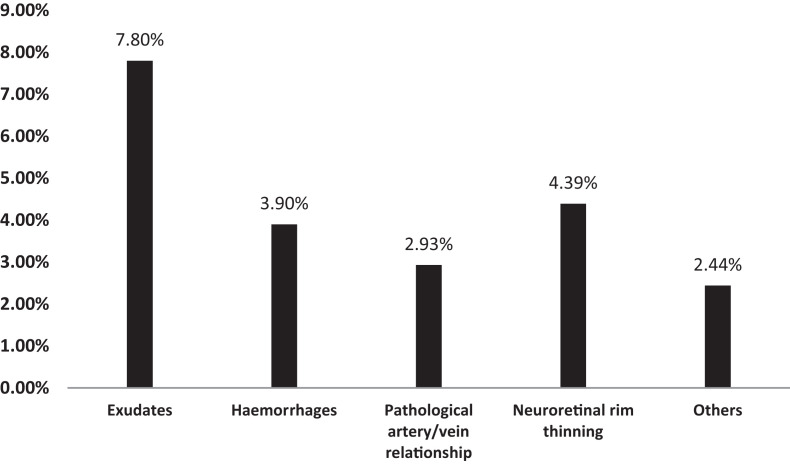
At least one type of fundus alteration was detected in 19 patients (18%; n = 19/104). Those 19 patients had a total of 31 eyes with lesions. The percentages for each type of lesion are shown in Figure 4, with a high presence of exudates (8%; n = 16/205) followed by hemorrhages (4%; n = 8/205). The two experts who reviewed each video independently agreed on the same lesion assessment in 87.9% of the videos and reached a consensus in 12.1% with initial independent assessments.
Figure 4.
Ocular lesions found by ophthalmologists during the examination of videos. Others = enhanced papillary excavation, lifting of vessels, tortuous vessels, cataract or vitritis, and multiple chorioretinal scars.
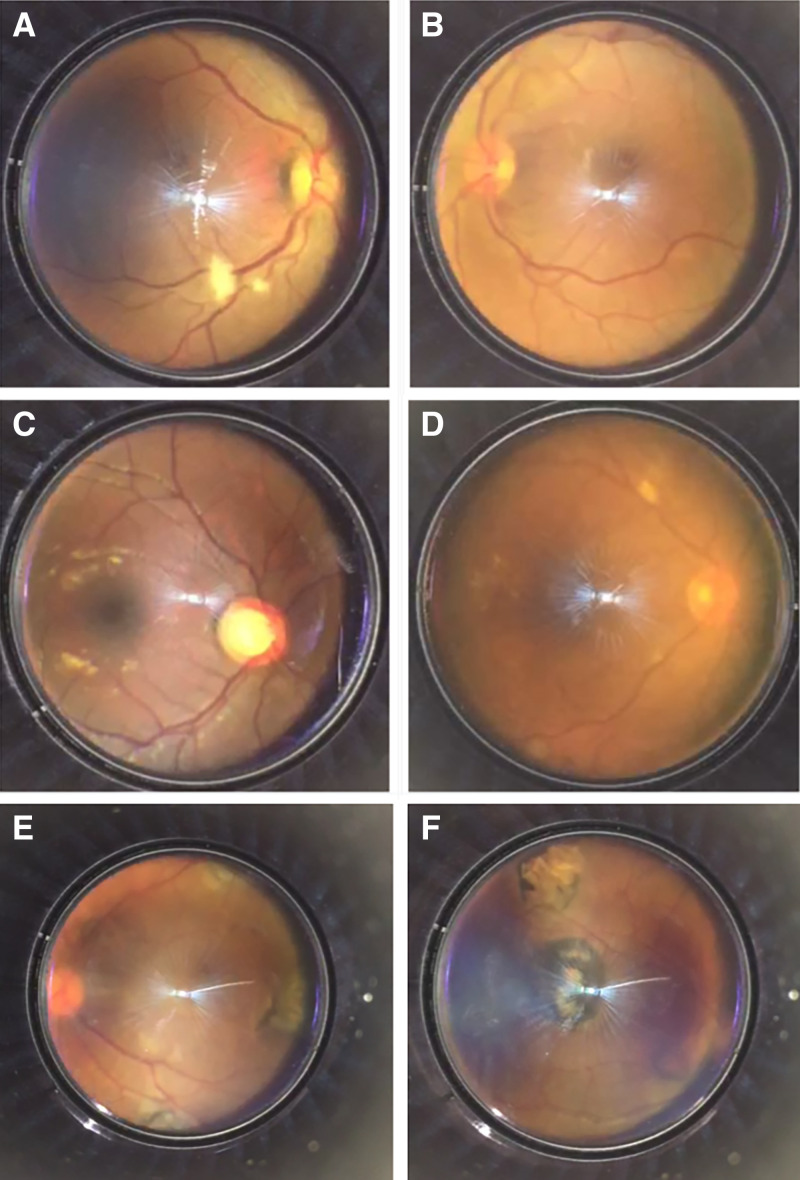
On the basis of the lesions found, experts agreed that there was probable pathology in 16 patients (15%; n = 16/104). Among the suspected ocular diseases were age-related macular degeneration, diabetic or hypertensive retinopathy or other vascular diseases, ocular toxoplasmosis, and retinal detachment. The images in Figure 5 illustrate some examples of the most common lesions found in the fundus videos acquired.
Figure 5.
Most common lesions found in the fundus videos acquired. (A) Exudate and pathological artery–vein relationship. (B) Hemorrhages. (C) Enhanced papillary excavation. (D) Exudates. (E and F) Multiple chorioretinal scars in a patient with suspected toxoplasmosis.
The presence or absence of ocular lesions by age and sex and in the population with known previous pathology (HIV, diabetes, hypertension) is shown in Table 1. Among patients with HIV, 19% had a lesion, with exudates being the most representative at 8%. This percentage increased to 37% in those without treatment and to 17% in patients with HIV and hypertension. Patients with previous hypertension or diabetes had elevated percentages of ocular lesions—31% and 25% (1 out of 4), respectively.
DISCUSSION
In this study, we present a low-cost retina screening device made of a 3D-printed plastic structure that can hold a lens and then be attached to a mobile phone. For this study, we used an iPhone 6 because of its optic design; other phones with similar designs could be used simply by changing one detachable piece of the device. The device includes an app for smartphones that enables the mobile phone to acquire retinal images and/or videos and send them to a telemedicine platform in the cloud. The device has been designed to allow the acquisition of images of the retinal fundus with very short and simple training. Features such as the short duration of the video, which facilitates its immediate visualization and delivery, makes it a potentially interesting and useful point-of-care tool.
Given that that the use of smartphones has been increasing rapidly in recent years, with more than 90% of the world’s population now owning a smartphone, we also expect that this trend will continue in the future and that smartphones will be widely available in LMICs.26,27 Because of this expansion, this portable technology is becoming more affordable, and it is an intuitive, easy-to-use solution, requiring only simple training but with big potential for scale. Further, 3D-printing technology allows the development of low-cost devices in a decentralized manner, something that can be of great utility for the local production of devices such as this one. By using low-cost materials and manufacturing processes, the device offers an affordable solution for rapid prototyping and streamlined production. Moreover, our approach to decentralized manufacturing allows for easier replacement of components, thereby reducing the overall carbon footprint. One of the notable 3D printers leveraged in this study is the BQ printer, a low-cost yet versatile device that is also suitable for industrial applications.
In our study, we set out to screen for ocular lesions in adult patients admitted to the general hospital of Manhiça, a high HIV-endemic area. The low discrepancy between experts in the identification of lesions and the high presence of lesions (18.3%) highlights the usefulness of tools such as this one that allows remote diagnosis through telemedicine platforms.
Clinical results from our study are coherent with previous research28,29 highlighting the relevance of screening for ophthalmological diseases, given the high percentage of lesions (more than one of every six patients screened) found in our study. The main lesions found were hemorrhages, pathological artery–vein relationship, and exudates.
A greater number of the lesions described in our study appeared in those patients with other coexisting morbidities. In fact, the high proportion of admitted HIV-infected patients with an ocular lesion in our study (19%) nearly doubled among those same patients not receiving antiretroviral treatment (37%) (Table 1). Nonetheless, our study was not designed to determine differences in these populations, and we have no data on the presence of other infections, in addition to a patient with suspected toxoplasmosis who had multiple chorioretinal scars. Future research aims to include this information about patients to make the statistical significance of the clinical results (device’s evaluation) more robust.
In this study, nonophthalmologists were collecting fundus videos with our device, and it was their first time using a retinograph. However, and remarkably, a short training of 3 hours was sufficient to instruct them on how to use it correctly, showing that 90% of the videos were of sufficient quality for diagnosis. In our study, we have also identified limitations with pupil dilation, fixation points, and patient collaboration that can interfere with adequate retinal structure visualization. To improve the study’s acquisition and repeatability, phenylephrine drops could be used with tropicamide for sufficient dilation. Nonmydriatic smartphone-based fundus cameras offer retinal visualization without discomfort, but their cost may hinder implementation in low-resource countries.18,22
Further research should be done to improve the usability and performance of the device and to get a significant sampling of data to assess the current ocular health of the Mozambique populations. In addition, it is essential to conduct comparison studies with other smartphone-based devices to benchmark the performance and efficiency of our 3D-printed retinograph. This comparative analysis will contribute to a comprehensive understanding of the device’s effectiveness and potential in various healthcare settings. Regarding data acquisition, automated analysis of retinal data could help scale-up population screening campaigns.30,31 Recently, artificial intelligence (AI) has been applied in the field of ophthalmology to improve diagnostic accuracy and automate image analysis. The capability of AI algorithms to analyze images makes it a useful tool for eye care that is integrated into day-to-day electronic devices, such as smartphones, which could offer a more affordable option and establish itself as a useful screening tool in ophthalmology. In fact, this type of technology combined with nonmydriatic cameras have shown great utility for screening the central retina’s diseases.32 There are a number of recently published public datasets composed of retinal fundus images that can be used to train and validate an AI algorithm, and this could be integrated with devices similar to the one presented in this article.8,22,33,34 The capability of AI algorithms to analyze images, combined with data from multiple sources, creates a powerful tool. AI and multimodal data acquisition have emerged as promising approaches in the field of medicine with the aim of improving diagnostic accuracy and automating image analysis.
CONCLUSION
General practitioners without previous experience in the use of retinographs successfully used a low-cost device manufactured through 3D printing and controlled by a smartphone. Furthermore, this study shows that this type of device could be a relevant and easy-to-use option in locations without available resources to conduct retinographies.
ACKNOWLEDGMENTS
ISGlobal receives support from the Spanish Ministry of Science and Innovation through the Centro de Excelencia Severo Ochoa 2019-2023 Program (CEX2018-000806-S) and support from the Generalitat de Catalunya through the CERCA Program. CISM is supported by the Government of Mozambique and the Spanish Agency for International Development. Spotlab from Bill & Melinda Gates Foundation.
REFERENCES
- 1. International Agency for the Prevention of Blindness , 2021. IAPB vision atlas. Available at: https://www.iapb.org/learn/vision-atlas/. Accessed August 3, 2023.
- 2. Pascolini D, Mariotti SP, 2012. Global estimates of visual impairment: 2010. Br J Ophthalmol 96: 614–618. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization , 2019. World Report on Vision. Geneva, Switzerland: WHO. [Google Scholar]
- 4. Nkanga D, Adenuga O, Okonkwo O, Ovienria W, Ibanga A, Agweye C, Oyekunle I, Akanbi T, 2020. Profile, visual presentation and burden of retinal diseases seen in ophthalmic clinics in sub-Saharan Africa. Clin Ophthalmol 14: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magan T, Pouncey A, Gadhvi K, Katta M, Posner M, Davey C, 2019. Prevalence and severity of diabetic retinopathy in patients attending the endocrinology diabetes clinic at Mulago Hospital in Uganda. Diabetes Res Clin Pract 152: 65–70. [DOI] [PubMed] [Google Scholar]
- 6. Khachatryan T, Mozaffar T, Mnatsakanyan L, 2022. Utility of video-fundoscopy and prospects of portable stereo-photography of the ocular fundus in neurological patients. BMC Neurol 22: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muiesan ML. et al. , 2017. Ocular fundus photography with a smartphone device in acute hypertension. J Hypertens 35: 1660–1665. [DOI] [PubMed] [Google Scholar]
- 8. Cheung CY. et al. , 2022. A deep learning model for detection of Alzheimer’s disease based on retinal photographs: a retrospective, multicentre case-control study. Lancet Digit Health 4: e806–e815. [DOI] [PubMed] [Google Scholar]
- 9. MacCormick IJ, Beare NA, Taylor TE, Barrera V, White VA, Hiscott P, Molyneux ME, Dhillon B, Harding SP, 2014. Cerebral malaria in children: using the retina to study the brain. Brain 137: 2119–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beare NAV, 2023. Cerebral malaria – using the retina to study the brain. Eye 37: 2379–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heiden D. et al. , 2007. Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med 4: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ford N. et al. , 2013. Burden of HIV-related cytomegalovirus retinitis in resource-limited settings: a systematic review. Clin Infect Dis 57: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 13. Bechange S, Jolley E, Virendrakumar B, Pente V, Milgate J, Schmidt E, 2020. Strengths and weaknesses of eye care services in sub-Saharan Africa: a meta-synthesis of eye health system assessments. BMC Health Serv Res 20: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quellec G, Bazin L, Cazuguel G, Delafoy I, Cochener B, Lamard M, 2016. Suitability of a low-cost, handheld, nonmydriatic retinograph for diabetic retinopathy diagnosis. Transl Vis Sci Technol 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, Niemeijer M, 2016. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci 57: 5200–5206. [DOI] [PubMed] [Google Scholar]
- 16. Bastawrous A. et al. , 2016. Clinical validation of a smartphone-based adapter for optic disc imaging in Kenya. JAMA Ophthalmol 134: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajalakshmi R, Prathiba V, Arulmalar S, Usha M, 2021. Review of retinal cameras for global coverage of diabetic retinopathy screening. Eye 35: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sengupta S, Sindal MD, Baskaran P, Pan U, Venkatesh R, 2019. Sensitivity and specificity of smartphone-based retinal imaging for diabetic retinopathy: a comparative study. Ophthalmol Retina 3: 146–153. [DOI] [PubMed] [Google Scholar]
- 19. Chalam KV, Chamchikh J, Gasparian S, 2022. Optics and utility of low-cost smartphone-based portable digital fundus camera system for screening of retinal diseases. Diagnostics (Basel) 12: 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim TN. et al. , 2021. Comparison of automated and expert human grading of diabetic retinopathy using smartphone-based retinal photography. Eye 35: 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaur R. et al. , 2020. MII RetCam assisted smartphone-based fundus imaging (MSFI) – a boon for paediatric retinal imaging. Eye 34: 1307–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malerbi FK. et al. , 2022. Diabetic retinopathy screening using artificial intelligence and handheld smartphone-based retinal camera. J Diabetes Sci Technol 16: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chandrakanth P, Gosalia H, Verghese S, Narendran K, Narendran V, 2022. The Gimbalscope – a novel smartphone-assisted retinoscopy technique. Indian J Ophthalmol 70: 3112–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. International Centre for Eye Health, Ministry of Health, Mozambique (MoHM), Olhos do Mundo (Mozambique), University of Cape Town , 2016. Mozambique—Inhambane Rapid Assessment of Avoidable Blindness Survey 2016. Grootebroek, the Netherlands: RAAB Repository. [Google Scholar]
- 25. Nhacolo A. et al. , 2021. Cohort profile update: Manhiça Health and Demographic Surveillance System (HDSS) of the Manhiça Health Research Centre (CISM). Int J Epidemiol 50: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kemp S, 2021. Digital 2021: Global Overview Report. DataReportal. Available at: https://datareportal.com/reports/digital-2021-global-overview-report. Accessed August 3, 2023.
- 27. International Telecommunications Union, Development Sector , 2021. Digital Development: Facts and Figures 2021. Available at: https://www.itu.int/en/ITU-D/Statistics/Documents/facts/FactsFigures2021.pdf. Accessed August 3, 2023.
- 28. Nkomazana O, Tshitswana D, 2008. Ocular complications of HIV infection in sub-Sahara Africa. Curr HIV/AIDS Rep 5: 120–125. [DOI] [PubMed] [Google Scholar]
- 29. Ekoru K. et al. , 2019. Type 2 diabetes complications and comorbidity in sub-Saharan Africans. EClinicalMedicine 16: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Medeiros FA, Jammal AA, Mariottoni EB, 2021. Detection of progressive glaucomatous optic nerve damage on fundus photographs with deep learning. Ophthalmology 128: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang X. et al. , 2022. Detecting glaucoma from multi-modal data using probabilistic deep learning. Front Med (Lausanne) 9: 923096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Font O, Torrents-Barrena J, Royo D, García SB, Zarranz-Ventura J, Bures A, Salinas C, Zapata M, 2022. Validation of an autonomous artificial intelligence-based diagnostic system for holistic maculopathy screening in a routine occupational health checkup context. Graefes Arch Clin Exp Ophthalmol 260: 3255–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panchal S, Naik A, Kokare M, Pachade S, Naigaonkar R, Phadnis P, Bhange A, 2023. Retinal Fundus Multi-Disease Image Dataset (RFMiD) 2.0: a dataset of frequently and rarely identified diseases. Data (Basel) 8: 29. [Google Scholar]
- 34. Nakahara K. et al. , 2022. Deep learning-assisted (automatic) diagnosis of glaucoma using a smartphone. Br J Ophthalmol 106: 587–592. [DOI] [PubMed] [Google Scholar]