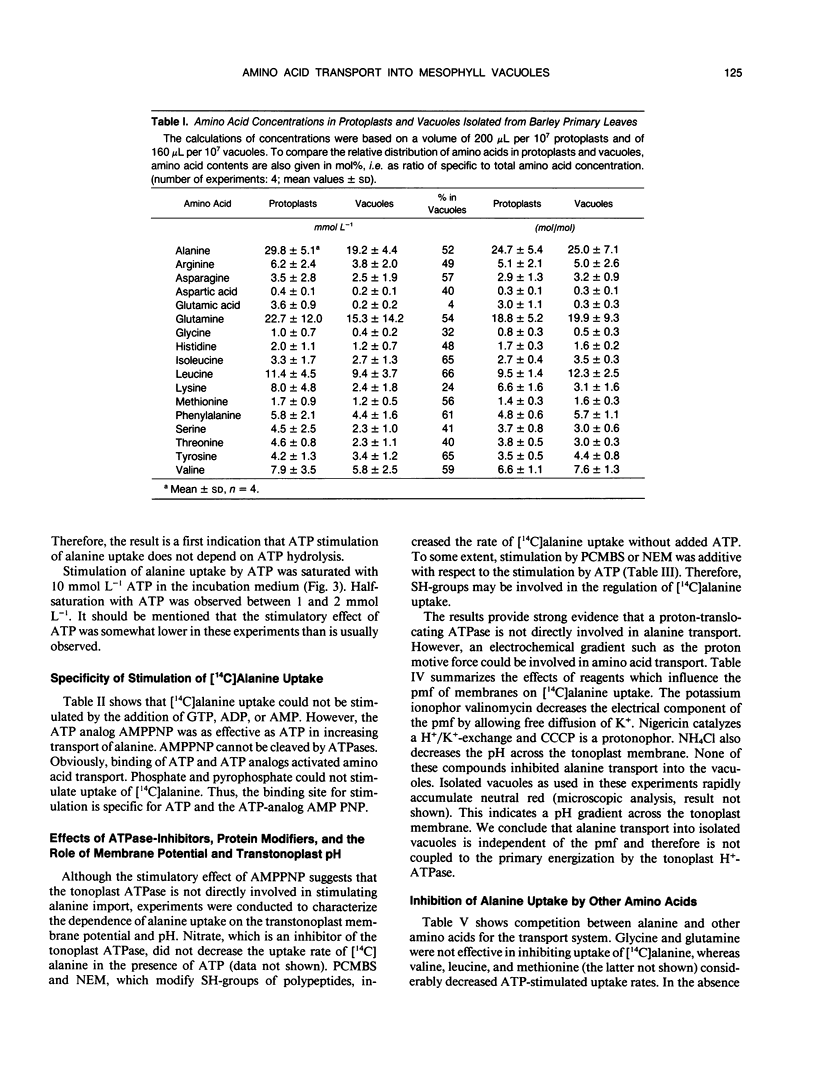
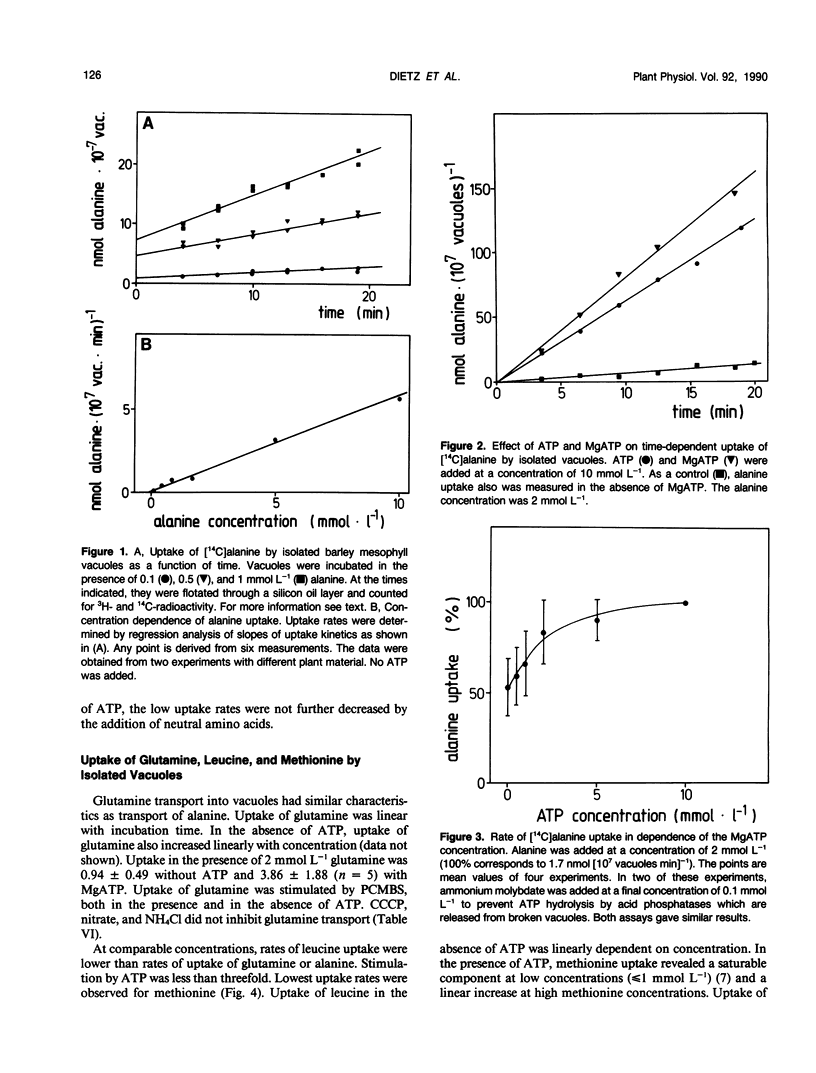
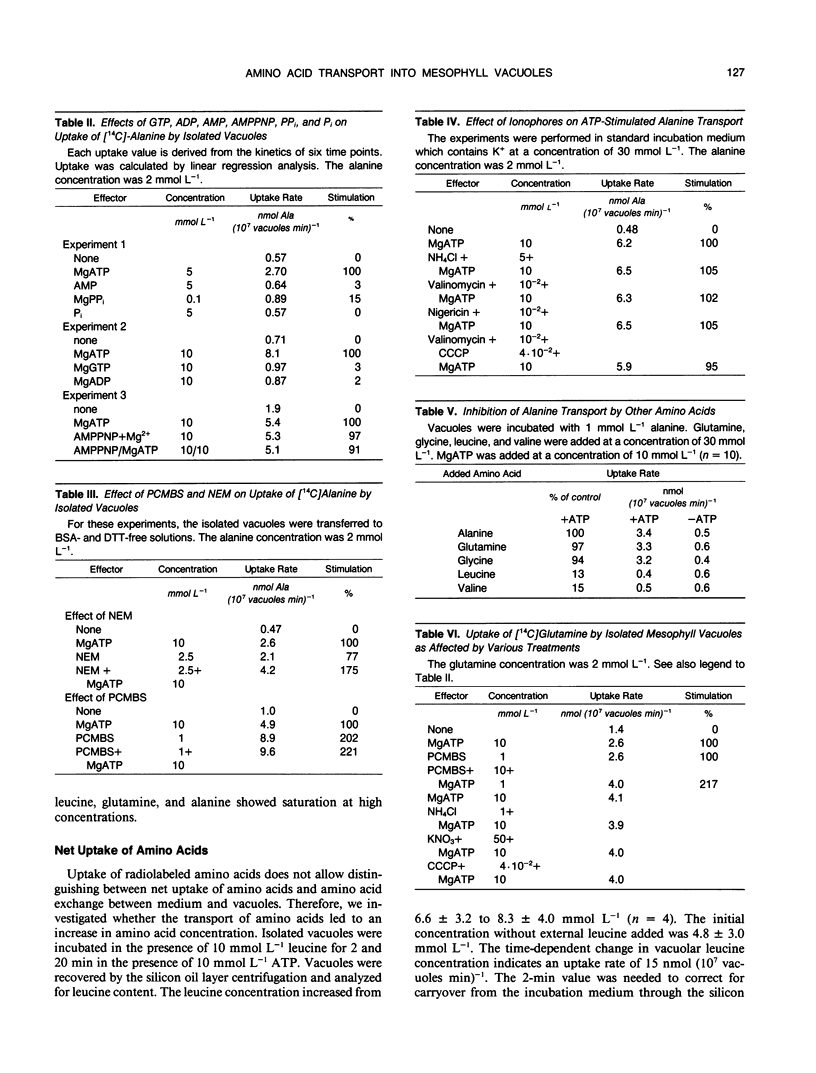
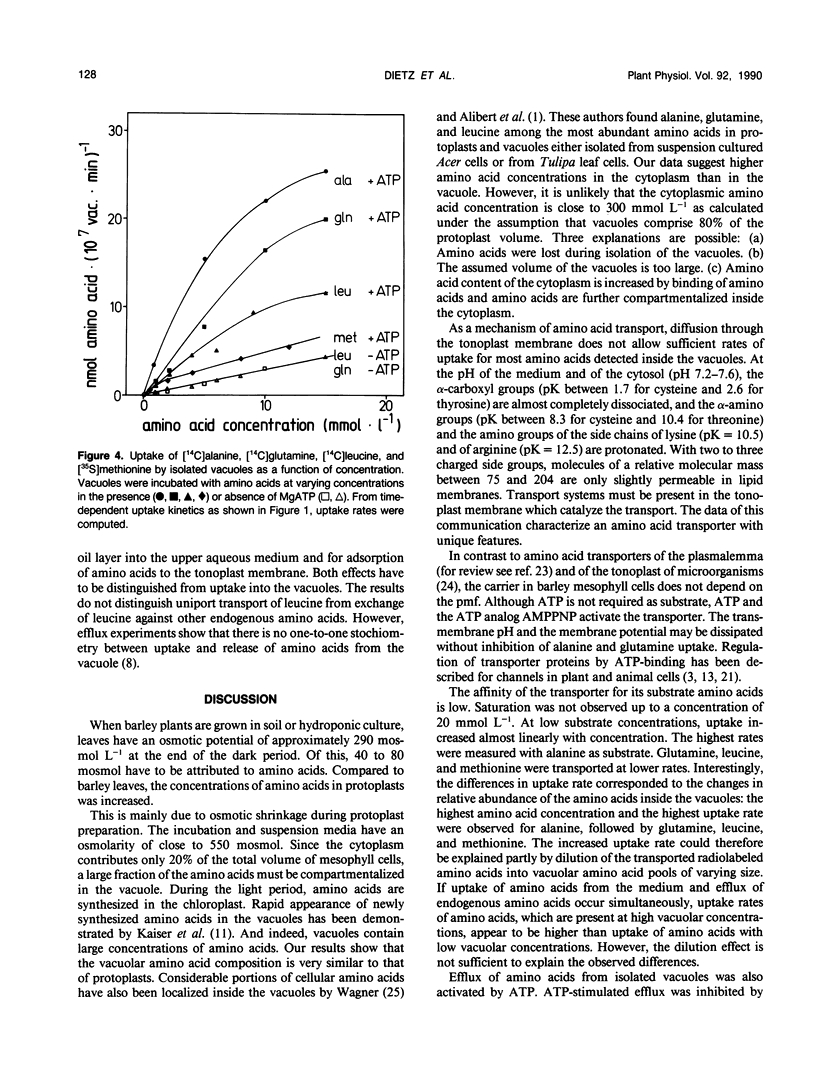
Abstract
Mesophyll protoplasts from leaves of well-fertilized barley (Hordeum vulgare L.) plants contained amino acids at concentrations as high as 120 millimoles per liter. With the exception of glutamic acid, which is predominantly localized in the cytoplasm, a major part of all other amino acids was contained inside the large central vacuole. Alanine, leucine, and glutamine are the dominant vacuolar amino acids in barley. Their transport into isolated vacuoles was studied using 14C-labeled amino acids. Uptake was slow in the absence of ATP. A three- to sixfold stimulation of uptake was observed after addition of ATP or adenylyl imidodiphosphate an ATP analogue not being hydrolyzed by ATPases. Other nucleotides were ineffective in increasing the rate of uptake. ATP-Stimulated amino acid transport was not dependent on the transtonoplast pH or membrane potential. p-Chloromercuriphenylsulfonic acid and n-ethyl maleimide increased transport independently of ATP. Neutral amino acids such as valine or leucine effectively decreased the rate of alanine transport. Glutamine and glycine were less effective or not effective as competitive inhibitors of alanine transport. The results indicate the existence of a uniport translocator specific for neutral or basic amino acids that is under control of metabolic effectors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Despeghel J. P., Delrot S. Energetics of Amino Acid Uptake by Vicia faba Leaf Tissues. Plant Physiol. 1983 Jan;71(1):1–6. doi: 10.1104/pp.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horák J. Amino acid transport in eucaryotic microorganisms. Biochim Biophys Acta. 1986 Dec 22;864(3-4):223–256. doi: 10.1016/0304-4157(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon S. L., Bown A. W. Evidence for a specific glutamate/h cotransport in isolated mesophyll cells. Plant Physiol. 1987 Mar;83(3):691–697. doi: 10.1104/pp.83.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel C. N., Holterman R. K., Bone R. F., Wozniak P. M. Amino Acid Transport in Suspension-Cultured Plant Cells : III. COMMON CARRIER SYSTEM FOR THE UPTAKE OF l-ARGININE, l-ASPARTIC ACID, l-HISTIDINE, l-LEUCINE, AND l-PHENYLALANINE. Plant Physiol. 1982 Jan;69(1):246–249. doi: 10.1104/pp.69.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. An arginine/histidine exchange transport system in vacuolar-membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1984 Sep 25;259(18):11509–11511. [PubMed] [Google Scholar]
- Wagner G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979 Jul;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]


