Abstract
Background and Purpose
Whether brain–heart communication continues under ventricular fibrillation (VF) remains to be determined. There is weak evidence of physiological changes in cortical activity under VF. Moreover, brain–heart communication has not previously been studied in this condition. We aimed to measure parallel changes in heart-rate variability (HRV), cortical activity, and brain–heart interactions in a patient who experienced VF.
Methods
The EEG and EKG signals for the case report were acquired for approximately 20 h. We selected different 1-min-long segments based on the changes in the EKG waveform. We present the changes in heartbeat-evoked responses (HERs), HRV, and EEG power for each selected segment.
Results
The overall physiological activity appeared to deteriorate as VF proceeded. Brain–heart interactions measured using HERs disappeared, with a few aberrant amplitudes appearing occasionally. The parallel changes in EEG and HRV were not pronounced, suggesting the absence of bidirectional neural control.
Conclusions
Our measurements of brain–heart interactions suggested that the evolving VF impairs communication between the central and autonomic nervous systems. These results may support that reduced brain–heart interactions reflect loss of consciousness and deterioration in the overall health state.
Keywords: ventricular fibrillation, consciousness, heartbeat-evoked responses, brain–heart interactions
INTRODUCTION
Ventricular fibrillation (VF) is a physiological condition characterized by turbulent and chaotic cardiac electrical activity that leads to irregular ventricular excitation.1 The effects of VF on cardiac activity include EKG changes in frequency, wave contour, and cardiac cycle amplitude.2 However, only a few studies have investigated VF physiology, and the effects on cortical activity have scarcely been described.3 One of the main consequences of VF is a very high heart rate that induces suboptimal blood supply,4 which leads to loss of consciousness and sudden cardiac death in prolonged episodes.1 Case reports have revealed the generalized slowing of EEG activity.5,6 The predominance of slow waves in EEG is associated with ongoing ischemia; that is, reduced oxygenation in the brain.7,8,9 Slowed EEG oscillations have been found in intermittent circulatory arrest10 and in patients who survived a cardiac arrest.11 The brain–heart interactions under ventricular tachycardia, and developing VF, have not previously been described in humans. There is some evidence on brain–heart interactions in animal models supporting that asphyxia stimulates brain–heart coupling, as measured by the spectral coherence between EEG and EKG signals.12 However, those relationships were revealed by analyzing the brain and EKG potential instead of functional interactions between the brain and heart rate.
The brain and heart communicate via different multisynaptic pathways. This bidirectional communication is mediated by the sympathetic and parasympathetic nervous systems.13 Specifically, the neural monitoring of visceral signals may contribute to mediate the awareness of the bodily state.14,15 In this case report, we describe the effects of VF on brain–heart interactions using EEG and EKG recordings. We tested the hypothesis that reduced oxygenation in the brain triggers changes in brain–heart coupling.12 We aimed to contribute to the knowledge of the brain and cardiac dynamics that remains poorly understood in the case of sudden arrhythmias.16
METHODS
Participant
This study included a case report of a female aged 66 years. The patient suffered a heart infarct, followed by VF and then death. The patient spent 11 days since the initial cardiac insult. The physiological recordings included 19-channel EEG and 1-lead EKG (MEDICID-05 system, Neuronic, S.A.; Havana, Cuba) performed for approximately 20 h continuously, at 200 Hz sampling frequency. The Review Board of the Institute of Neurology and Neurosurgery (Havana, Cuba) approved this study (IRB No. n2023-AA). Written consent was obtained from the proxy of the patient, as required by the Declaration of Helsinki.
Data preprocessing
Data processing was performed using the FieldTrip toolbox (Nijmegen, the Netherlands) in MATLAB.17 EEG data were offline filtered using a 1–45 Hz Butterworth fourth-order bandpass filter. Different 1-min segments of EEG data were selected manually for further analysis based on the EKG patterns. The criteria for selecting the EKG segments were 1) a cycle-like pattern to allow locking EEG to the cardiac cycle during the heartbeat-evoked responses (HERs) analysis and 2) each EKG pattern was present for at least 1 min. These criteria identified 19 segments: 7 normal sinus rhythms and 12 during the ongoing VF. Muscle, eye-movement, and cardiac-field artifacts were removed using independent-components analysis, and were consecutively re-referenced using a common average.18 The EEG spectrogram was computed using the short-time Fourier transform with a 2-s sliding time window and a 50% overlap. Time-varying EEG oscillations were integrated within the delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–45 Hz) bands.
Physiological markers
Heart-rate variability
Heartbeats were detected in EKG using an automated process based on a sliding time window that detected R-peaks. Both peak detection and the resulting histogram of the interbeat-interval duration were visually inspected to correct misdetections.18 The heart-rate variability (HRV) was analyzed in the time and frequency domains using a smoothed pseudo-Wigner–Ville distribution19 to obtain the time-varying low frequency (0.04–0.15 Hz), high frequency (0.15–0.4 Hz), and very high frequency (0.4–0.9 Hz) components.
Heartbeat-evoked responses analysis
HERs were defined by time-locking EEG epochs relative to the R-peaks.20 Epochs with amplitudes >300 µV in any channel were discarded. The latency of the computed HERs were computed at -100 ms relative to the R-peak, up to 500 ms. If one EEG epoch was associated with an interbeat interval shorter than 500 ms, it was not included int the HER computation. If more than 20% of the EEG epochs were discarded because of the interbeat interval duration, the latency was redefined to preserve at least 80% of them.
RESULTS
We computed physiological markers from 20-h EEG and EKG recordings made in a patient who developed VF. We manually selected 19 1-min time windows: 7 during the normal sinus period and 12 from the beginning of pattern changes on the EKG (note that only 35 s are reported for the last VF period).
Fig. 1 shows the different time windows with a normal sinus rhythm in EKG. The physiological activity exhibited no aberrant changes according to measurements of EEG power, HRV, and HERs. HERs appear to present a stable pattern, with a midline positivity at 0.1–0.2 s relative to the R-peak. Fig. 2 shows different EKG patterns selected during the development of ventricular tachycardia. The first time window (e0) was approximately 20 h before the VF offset. Twelve EKG patterns were identified during the last 70 min (ei, i = 1, …, 12) of the recording, based on the changes in EKG readings.
Fig. 1. EKG patterns, EEG power, HRV, and HERs for 60-s periods at different times before the EKG pattern changed, which developed into ventricular tachycardia (at approximately t=66,800 s). The onset time windows were t1=500 s, t2=5,000 s, t3=10,000 s, t4=15,000 s, t5=20,000 s, t6=40,000 s, and t7=60,000 s. HER, heartbeat-evoked response; HF, high frequency; HRV, heart-rate variability; IBI, interbeat interval; LF, low frequency; VHF, very high frequency.
Fig. 2. EKG signals of the patient in the last 70 min relative to the VF offset. Distinctive EKG patterns were identified for further analysis. VF, ventricular fibrillation.
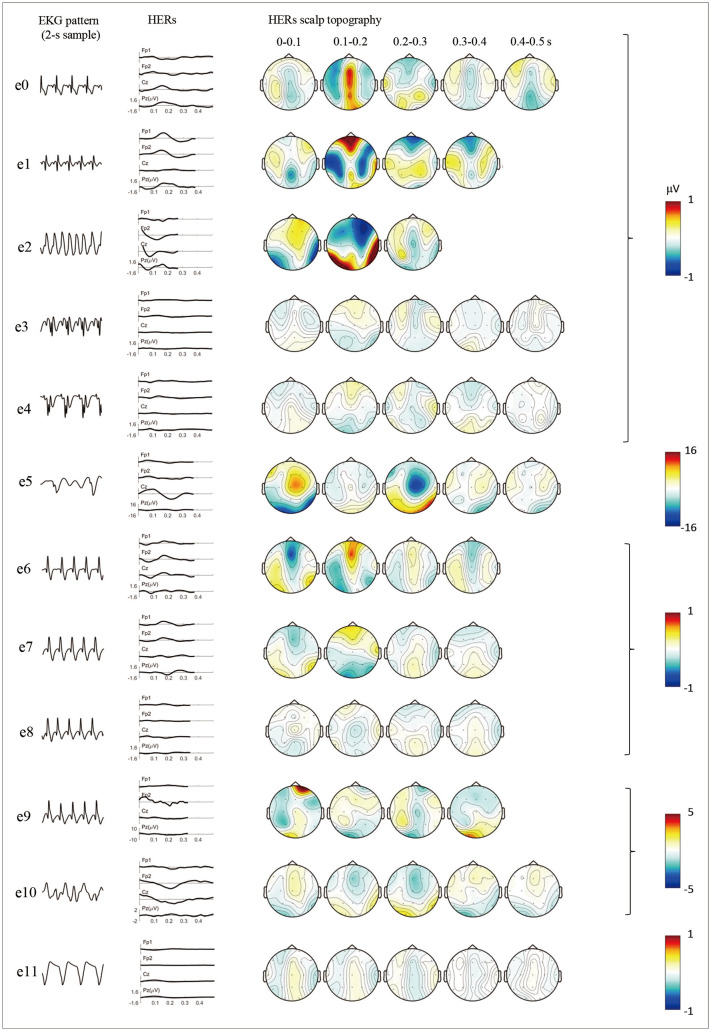
Fig. 3 shows the evolution of the brain–heart interactions as measured by HERs. HER positivity was present in midline channels at 0.1–0.2 s during the normal sinus period (e0). HERs remained prominent in prefrontal channels when ventricular tachycardia began (e1), and disappeared completely in the subsequent periods. During the e5, e9, and e10 patterns, there was a substantial increase in the observed amplitudes of the HERs.
Fig. 3. Changes in HERs. The first column shows a 2-s sample of the EKG pattern and the second shows the time course of HERs in four EEG channels: Fp1, Fp2, Cz, and Pz. The third column shows the changes in the scalp topography of HERs with a 0.1-s step. HER, heartbeat-evoked response.
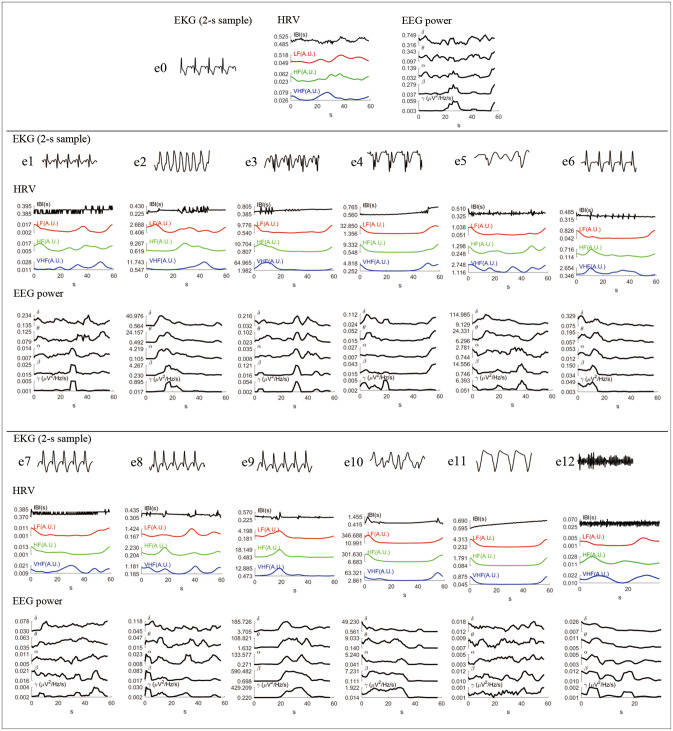
Fig. 4 shows the time-varying HRV and EEG power for all the studied samples in chronological order. The first row shows the period in which the subject had a normal sinus rhythm, which did not present visually aberrant oscillations on HRV nor EEG. The patient lost HRV and EEG activity as the VF evolved. However, there was a substantial increase in EEG power during e2, e5, and e10, specifically in the delta band. In a few cases, changes in HRV were correlated with or preceded changes in EEG power (see e3, e6, and e9).
Fig. 4. Changes in HRV and EEG power, from normal sinus (e0) to the final ventricular fibrillation (e12). The first row shows a 2-s sample of the EKG. The second row shows the changes in HRV as measured using the IBI and in the LF, HF, and VHF bands. The third row shows the time-varying EEG power in five frequency bands. The power was averaged among all EEG channels. HF, high frequency; HRV, heart-rate variability; IBI, interbeat interval; LF, low frequency; VHF, very high frequency.
DISCUSSION
This study aimed to characterize the brain–heart interactions under VF. Previous data from animal models provide evidence of strong brain–heart coupling in corticocardiac voltage signals when the heart has deteriorated markedly during asphyxia.12 Because VF causes reduced oxygenation as a result of a suboptimal blood supply,4 we tested the hypothesis of strong functional brain–heart coupling in a human case of VF development.
The overall electrophysiological activities in both the brain and heart deteriorated as the VF evolved. Such deteriorated neural activity was also reflected in the HERs, as the amplitude decreased as VF evolved. There were short-term increases in the observed HER amplitudes, most likely due to an increase in delta waves.21 We did not observe an aberrant increase in the covarying activity between EEG oscillations and HRV components. HRV was substantially reduced in the last periods before VF.
These results indicate that EEG and cardiac activity were uncoupled during VF development, contrary to suggestions based on results obtained in an animal model of asphyxia.12 Our results suggest that the increased brain–heart coupling observed in the animal model of asphyxia is related to an increase in the number of cardiac field artifacts in the measured brain data.22 The disappearance of HERs suggested that cardiac activity monitoring had ceased, given that HERs have been found to be associated with the neural processing of cardiac activity in multiple studies.20 This also suggests that loss of consciousness is reflected in HERs.21,23 However, it remains to be confirmed if low-amplitude HERs are related to loss of consciousness or the overall deterioration in the health state. The HERs results may also reflect the disappearance of the cardiac-field artifacts during VF.22 However, we corrected the cardiac-field artifacts in EEG using independent-components analysis, as recommended to avoid biased measurements in HERs.24
The present findings highlight the importance of understanding brain–heart interactions in pathological conditions that affect either the brain or the heart. For example, cerebrovascular accidents may be linked to ischemic attacks with cardiac arrhythmias,25 and sudden cardiac death may be caused by severe brain damage.26 HERs are potential biomarkers reflecting cognitive and interoceptive impairments under various neurodegenerative conditions.27,28 Our results confirm and highlight the importance of studying brain–heart interactions under cardiac pathologies, such as the recently observed reduction of HERs in patients with atrial fibrillation29 and cardiac arrest.30 Understanding the mechanisms of brain–heart interactions will contribute to utilizing EEG in prognosis of postcomatose conditions related to cardiac failure.31,32,33,34 Malfunction of the brain–heart communication pathways can damage the structure and function of the heart.35 Indeed, some mechanisms in which different autonomic activations can cause either arrhythmogenic or antiarrhythmic activity have already been described; for example, VF can be triggered by proarrhythmic sympathetic and antiarrhythmic parasympathetic activations.36 Cardiac arrhythmias leading to VF can also be caused by anatomical conditions, such as increased sympathetic nerve sprouting and regional myocardial hyperinnervation.37 Understanding the ongoing mechanisms that cause VF may facilitate the development of novel treatments, such as external modulations of the autonomic tone to reduce cardiac arrhythmia risk, with potential testing on animal models of resuscitation,38 postinfarction heart failure,37 and asphyxia.12
Parallel observations of EEG and autonomic activity have previously been made in case reports with near-death outcomes. The main observations were that brain inactivity preceded autonomic measurements, while observations of the presence of delta waves after death according to neurological criteria and the cessation of autonomic marker presentation are rare.39 Another study found that cortical activity cessation occurred in parallel with either the last QRS complex or cerebral blood flow cessation.40 The existing evidence from human case reports indicates that EEG cannot be used to predict the cardiac arrest period.39 The present study has confirmed that VF cannot be anticipated by monitoring EEG activity, and increased numbers of delta waves occur after the appearance of EKG patterns that develop into ventricular tachycardia.
Numerous confounding factors have been identified in brain–heart interaction studies.20,24,41 These factors can be controlled by simultaneously monitoring other physiological activities, such as the breathing rate, temperature, skin conductance, and blood pressure. Unfortunately, these physiological recordings were not available for this case report, which constituted the main limitation of our study.
The characterization of neural dynamics in near-death contexts may have implications in consciousness research,42 specifically in understanding the role of brain–heart interactions in consciousness,15 but also in understanding the cognitive processes in near-death experiences.43,44,45
Footnotes
- Conceptualization: Diego Candia-Rivera, Calixto Machado.
- Data curation: Calixto Machado.
- Formal analysis: Diego Candia-Rivera, Calixto Machado.
- Investigation: Diego Candia-Rivera, Calixto Machado.
- Methodology: Diego Candia-Rivera.
- Resources: Calixto Machado.
- Software: Diego Candia-Rivera.
- Supervision: Calixto Machado.
- Visualization: Diego Candia-Rivera.
- Writing—original draft: Diego Candia-Rivera.
- Writing—review & editing: Diego Candia-Rivera, Calixto Machado.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Jalife J. Ventricular fibrillation: mechanisms of initiation and maintenance. Annu Rev Physiol. 2000;62:25–50. doi: 10.1146/annurev.physiol.62.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Reed MJ, Clegg GR, Robertson CE. Analysing the ventricular fibrillation waveform. Resuscitation. 2003;57:11–20. doi: 10.1016/s0300-9572(02)00441-0. [DOI] [PubMed] [Google Scholar]
- 3.Taggart P. Brain-heart interactions and cardiac ventricular arrhythmias. Neth Heart J. 2013;21:78–81. doi: 10.1007/s12471-012-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray RA, Jalife J, Panfilov AV, Baxter WT, Cabo C, Davidenko JM, et al. Mechanisms of cardiac fibrillation. Science. 1995;270:1222–1223. [PubMed] [Google Scholar]
- 5.Biesbroek JM, Hopmans EM, Seeber AA, Tromp SC. EEG registration during ventricular tachycardia and resuscitation. Neurol Clin Pract. 2018;8:e7–e8. doi: 10.1212/CPJ.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallmon H, Weber SC, Berger F, Will JC. Unexpected ventricular tachycardia following acoustic provocation during electroencephalography. Arch Dis Child. 2021;106:708. doi: 10.1136/archdischild-2020-320420. [DOI] [PubMed] [Google Scholar]
- 7.de Vries JW, Bakker PF, Visser GH, Diephuis JC, van Huffelen AC. Changes in cerebral oxygen uptake and cerebral electrical activity during defibrillation threshold testing. Anesth Analg. 1998;87:16–20. doi: 10.1097/00000539-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 8.de Vries JW, Visser GH, Bakker PF. Neuromonitoring in defibrillation threshold testing. A comparison between EEG, near-infrared spectroscopy and jugular bulb oximetry. J Clin Monit. 1997;13:303–307. doi: 10.1023/a:1007323823806. [DOI] [PubMed] [Google Scholar]
- 9.Singer I, van der Laken J, Edmonds HL, Jr, Slater AD, Austin E, Shields CB, et al. Is defibrillation testing safe? Pacing Clin Electrophysiol. 1991;14(11 Pt 2):1899–1904. doi: 10.1111/j.1540-8159.1991.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 10.Vriens EM, Bakker PF, Vries JW, Wieneke GH, Van Huffelen AC. The impact of repeated short episodes of circulatory arrest on cerebral function. Reassuring electroencephalographic (EEG) findings during defibrillation threshold testing at defibrillator implantation. Electroencephalogr Clin Neurophysiol. 1996;98:236–242. doi: 10.1016/0013-4694(95)00248-0. [DOI] [PubMed] [Google Scholar]
- 11.Lemmi H, Hubbert CH, Faris AA. The electroencephalogram after resuscitation of cardiocirculatory arrest. J Neurol Neurosurg Psychiatry. 1973;36:997–1002. doi: 10.1136/jnnp.36.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Mabrouk OS, Liu T, Tian F, Xu G, Rengifo S, et al. Asphyxia-activated corticocardiac signaling accelerates onset of cardiac arrest. Proc Natl Acad Sci U S A. 2015;112:E2073–E2082. doi: 10.1073/pnas.1423936112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WG, Schloesser D, Arensdorf AM, Simmons JM, Cui C, Valentino R, et al. The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. 2021;44:3–16. doi: 10.1016/j.tins.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzalini D, Rebollo I, Tallon-Baudry C. Visceral signals shape brain dynamics and cognition. Trends Cogn Sci. 2019;23:488–509. doi: 10.1016/j.tics.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Candia-Rivera D. Brain-heart interactions in the neurobiology of consciousness. Curr Res Neurobiol. 2022;3:100050. doi: 10.1016/j.crneur.2022.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou R, Shi W, Tao J, Li H, Lin X, Yang S, et al. Neurocardiology: cardiovascular changes and specific brain region infarcts. Biomed Res Int. 2017;2017:5646348. doi: 10.1155/2017/5646348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candia-Rivera D, Catrambone V, Valenza G. The role of electroencephalography electrical reference in the assessment of functional brain-heart interplay: from methodology to user guidelines. J Neurosci Methods. 2021;360:109269. doi: 10.1016/j.jneumeth.2021.109269. [DOI] [PubMed] [Google Scholar]
- 19.Orini M, Bailón R, Mainardi LT, Laguna P, Flandrin P. Characterization of dynamic interactions between cardiovascular signals by time-frequency coherence. IEEE Trans Biomed Eng. 2012;59:663–673. doi: 10.1109/TBME.2011.2171959. [DOI] [PubMed] [Google Scholar]
- 20.Park HD, Blanke O. Heartbeat-evoked cortical responses: underlying mechanisms, functional roles, and methodological considerations. Neuroimage. 2019;197:502–511. doi: 10.1016/j.neuroimage.2019.04.081. [DOI] [PubMed] [Google Scholar]
- 21.Candia-Rivera D, Machado C. TH-108. assessing the preservation of brain-heart communication in disorders of consciousness: comparing EEG locked to heartbeats vs. EEG non-locked to heartbeats. Clin Neurophysiol. 2022;141:S110 [Google Scholar]
- 22.Dirlich G, Vogl L, Plaschke M, Strian F. Cardiac field effects on the EEG. Electroencephalogr Clin Neurophysiol. 1997;102:307–315. doi: 10.1016/s0013-4694(96)96506-2. [DOI] [PubMed] [Google Scholar]
- 23.Candia-Rivera D, Annen J, Gosseries O, Martial C, Thibaut A, Laureys S, et al. Neural responses to heartbeats detect residual signs of consciousness during resting state in postcomatose patients. J Neurosci. 2021;41:5251–5262. doi: 10.1523/JNEUROSCI.1740-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buot A, Azzalini D, Chaumon M, Tallon-Baudry C. Does stroke volume influence heartbeat evoked responses? Biol Psychol. 2021;165:108165. doi: 10.1016/j.biopsycho.2021.108165. [DOI] [PubMed] [Google Scholar]
- 25.Pyner S. The paraventricular nucleus and heart failure. Exp Physiol. 2014;99:332–339. doi: 10.1113/expphysiol.2013.072678. [DOI] [PubMed] [Google Scholar]
- 26.Silvani A, Calandra-Buonaura G, Dampney RA, Cortelli P. Brain-heart interactions: physiology and clinical implications. Philos Trans A Math Phys Eng Sci. 2016;374:20150181. doi: 10.1098/rsta.2015.0181. [DOI] [PubMed] [Google Scholar]
- 27.Salamone PC, Legaz A, Sedeño L, Moguilner S, Fraile-Vazquez M, Campo CG, et al. Interoception primes emotional processing: multimodal evidence from neurodegeneration. J Neurosci. 2021;41:4276–4292. doi: 10.1523/JNEUROSCI.2578-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salamone PC, Esteves S, Sinay VJ, García-Cordero I, Abrevaya S, Couto B, et al. Altered neural signatures of interoception in multiple sclerosis. Hum Brain Mapp. 2018;39:4743–4754. doi: 10.1002/hbm.24319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumral D, Al E, Cesnaite E, Kornej J, Sander C, Hensch T, et al. Attenuation of the heartbeat-evoked potential in patients with atrial fibrillation. JACC Clin Electrophysiol. 2022;8:1219–1230. doi: 10.1016/j.jacep.2022.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Schulz A, Stammet P, Dierolf AM, Vögele C, Beyenburg S, Werer C, et al. Late heartbeat-evoked potentials are associated with survival after cardiac arrest. Resuscitation. 2018;126:7–13. doi: 10.1016/j.resuscitation.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Choi WJ, Lee JH, Kim SH. Neurological prognostication using raw EEG patterns and spectrograms of frontal EEG in cardiac arrest patients. J Clin Neurophysiol. 2020;39:427–433. doi: 10.1097/WNP.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 32.Hofmeijer J, Tjepkema-Cloostermans MC, van Putten MJ. Burst-suppression with identical bursts: a distinct EEG pattern with poor outcome in postanoxic coma. Clin Neurophysiol. 2014;125:947–954. doi: 10.1016/j.clinph.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Rossetti AO, Carrera E, Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology. 2012;78:796–802. doi: 10.1212/WNL.0b013e318249f6bb. [DOI] [PubMed] [Google Scholar]
- 34.Ruijter BJ, Tjepkema-Cloostermans MC, Tromp SC, van den Bergh WM, Foudraine NA, Kornips FHM, et al. Early electroencephalography for outcome prediction of postanoxic coma: a prospective cohort study. Ann Neurol. 2019;86:203–214. doi: 10.1002/ana.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuels MA. The brain-heart connection. Circulation. 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- 36.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 37.Zipes DP. Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleve Clin J Med. 2008;75 Suppl 2:S94–S96. doi: 10.3949/ccjm.75.suppl_2.s94. [DOI] [PubMed] [Google Scholar]
- 38.Schramm AE, Carton-Leclercq A, Diallo S, Navarro V, Chavez M, Mahon S, et al. Identifying neuronal correlates of dying and resuscitation in a model of reversible brain anoxia. Prog Neurobiol. 2020;185:101733. doi: 10.1016/j.pneurobio.2019.101733. [DOI] [PubMed] [Google Scholar]
- 39.Norton L, Gibson RM, Gofton T, Benson C, Dhanani S, Shemie SD, et al. Electroencephalographic recordings during withdrawal of life-sustaining therapy until 30 minutes after declaration of death. Can J Neurol Sci. 2017;44:139–145. doi: 10.1017/cjn.2016.309. [DOI] [PubMed] [Google Scholar]
- 40.Matory AL, Alkhachroum A, Chiu WT, Eliseyev A, Doyle K, Rohaut B, et al. Electrocerebral signature of cardiac death. Neurocrit Care. 2021;35:853–861. doi: 10.1007/s12028-021-01233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Candia-Rivera D, Sappia MS, Horschig JM, Colier WNJM, Valenza G. Confounding effects of heart rate, breathing rate, and frontal fNIRS on interoception. Sci Rep. 2022;12:20701. doi: 10.1038/s41598-022-25119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parnia S, Fenwick P. Near death experiences in cardiac arrest: visions of a dying brain or visions of a new science of consciousness. Resuscitation. 2002;52:5–11. doi: 10.1016/s0300-9572(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 43.Greyson B, van Lommel P, Fenwick P. Commentary: enhanced interplay of neuronal coherence and coupling in the dying human brain. Front Aging Neurosci. 2022;14:899491. doi: 10.3389/fnagi.2022.899491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Lommel P. Near-death experiences: the experience of the self as real and not as an illusion. Ann N Y Acad Sci. 2011;1234:19–28. doi: 10.1111/j.1749-6632.2011.06080.x. [DOI] [PubMed] [Google Scholar]
- 45.Vicente R, Rizzuto M, Sarica C, Yamamoto K, Sadr M, Khajuria T, et al. Enhanced interplay of neuronal coherence and coupling in the dying human brain. Front Aging Neurosci. 2022;14:813531. doi: 10.3389/fnagi.2022.813531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.