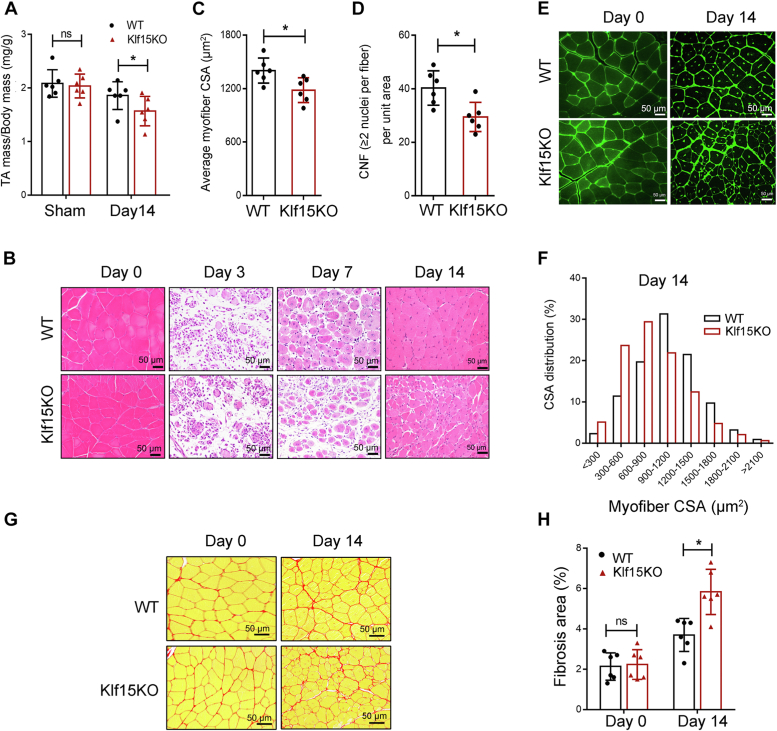
Figure 2.
Impaired muscle repair in Klf15KO mice upon injury. CTX was injected into the TA muscles, and the mice were then sacrificed at different time points as indicated after injury. A, TA muscle weight normalized to body weight at day 14 after injury (n = 6, ∗p < 0.05, ns, no significant difference, unpaired student t test). B, representative H&E-stained TA muscles illustrating the course of regeneration in WT and Klf15KO mice (n = 4–6). C, quantification of average cross-sectional area (CSA) of regenerating myofibers at day 14 postinjury (n = 6, ∗p < 0.05, unpaired student t test). D, number of myofibers containing two or more centrally located nuclei per field at day 7 postinjury (n = 6, ∗p < 0.05, unpaired student t test). E, representative WGA-stained TA muscles from WT and Klf15KO mice illustrating the size of myofiber at day 14 post CTX injury (n = 6). F, the distribution of myofiber sizes in WT and Klf15KO mice was analyzed from the CSA of ∼250 myofibers of each sample. G, Picro-Sirius red–stained TA muscles showed the collagen deposition at days 0 and 14 after injury. H, quantitative analysis of the area of positive Picro-Sirius red staining in each group (n = 6, ∗p < 0.05, ns, no significant difference, unpaired student t test). CTX, cardiotoxin; KLF15, Krüppel-like factor 15.