Abstract
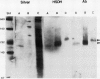
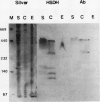
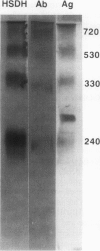
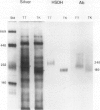
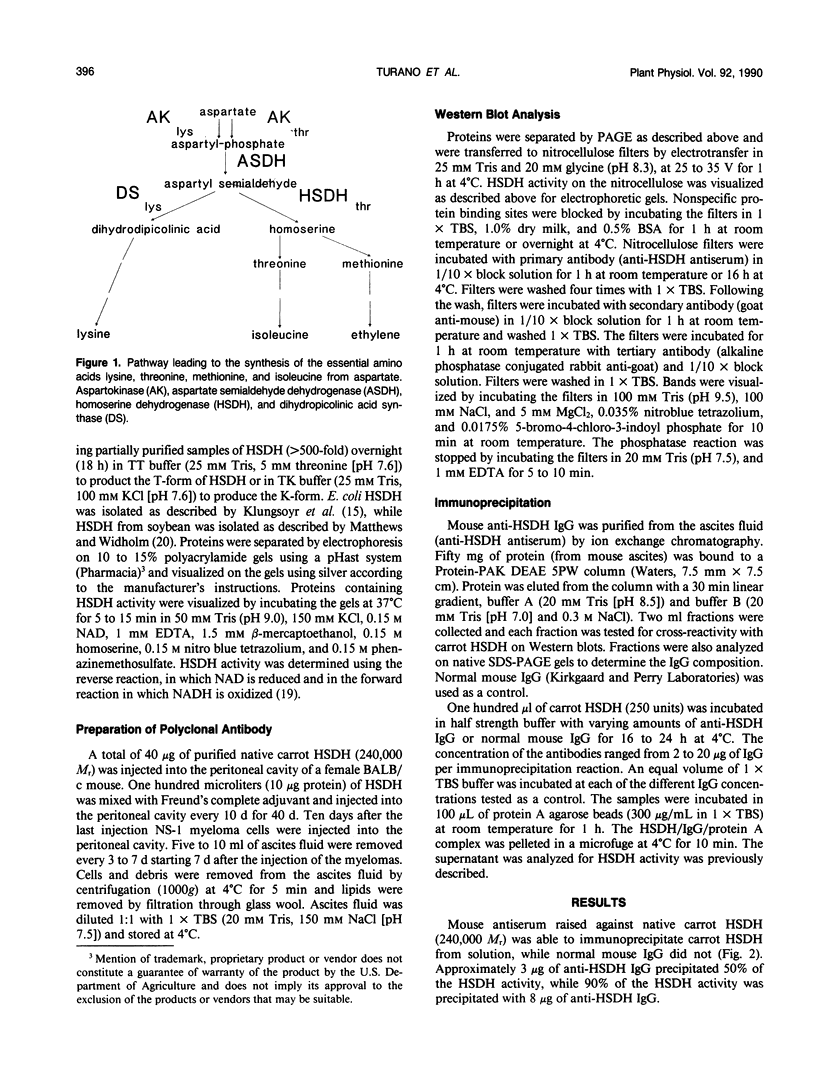
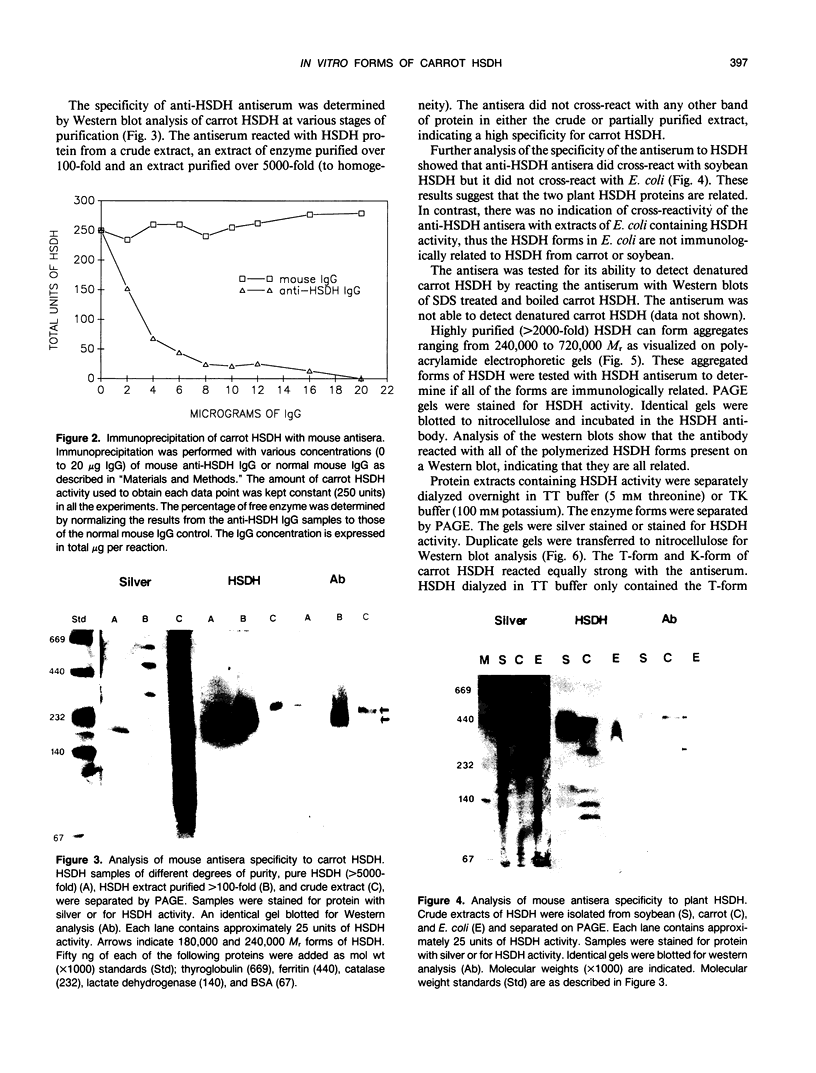
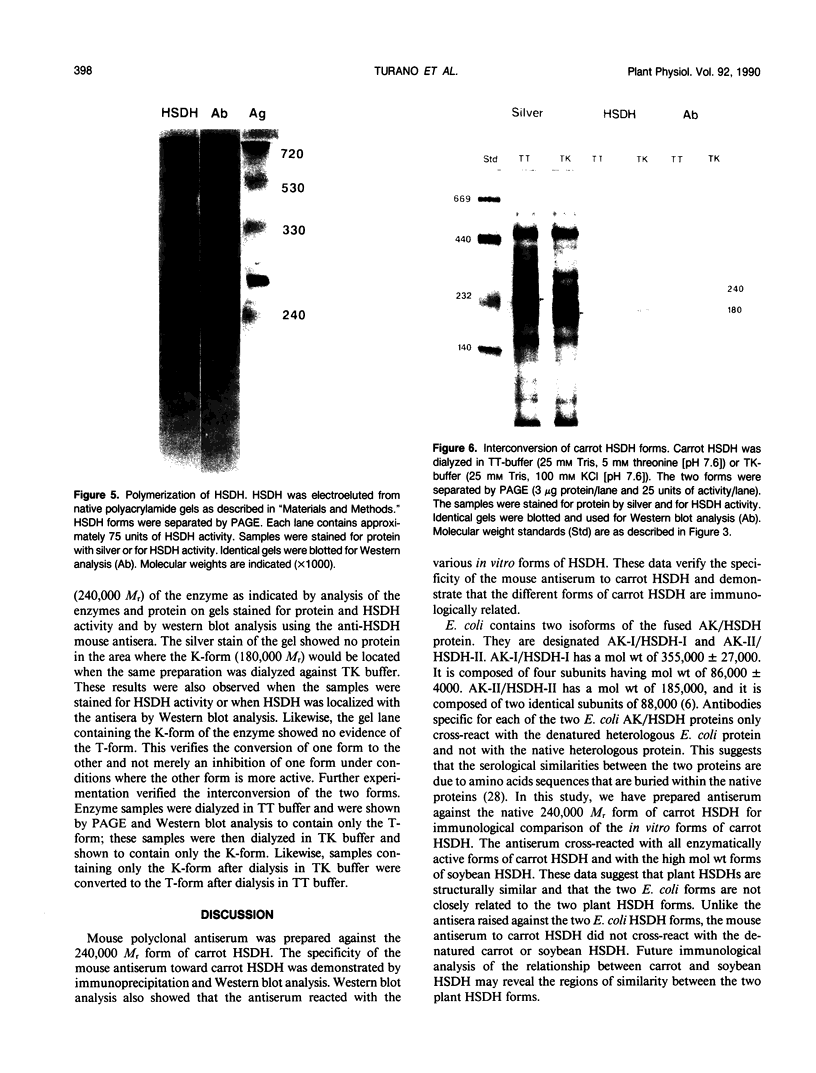
Multiple forms of homoserine dehydrogenase (HSDH) from carrot (Daucus carota L.) have been identified. One form of HSDH (T-form) has a relative molecular weight of 240,000 and is strongly inhibited by threonine. Another form (K-form) has a relative molecular weight of 180,000 and is insensitive to inhibition by threonine. The interconversion of these two forms is dependent upon the presence or absence of threonine and potassium. Polyacrylamide electrophoretic gels stained for HSDH activity and protein, paralleled with Western blot analysis, verified the interconversion of the T- and K-forms in 5 millimolar threonine and 100 millimolar potassium, respectively. Carrot HSDH also aggregates to form higher molecular weight complexes of 240,000 up to 720,000 Mr. Polyclonal antibody from mouse was raised against the T-form (240,000 Mr) of carrot HSDH. Specificity of the mouse antisera to carrot HSDH was verified by immunoprecipitation and Western blot analysis. The T-form, K-form, and all of the higher molecular aggregates of carrot HSDH cross-reacted with the anti-HSDH antiserum. The antiserum also cross-reacted with soybean HSDH, but did not cross-react with either of the two HSDH forms found in Escherichia coli. A model for the in vivo regulation of threonine biosynthesis in the chloroplast is presented. The model is based on the interconversion of the HSDH forms by potassium and threonine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan J. K., Lissik E. A., Matthews B. F. Changes in Enzyme Regulation during Growth of Maize: III. Intracellular Localization of Homoserine Dehydrogenase in Chloroplasts. Plant Physiol. 1977 Apr;59(4):673–679. doi: 10.1104/pp.59.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA P., GEST H., SEGAL H. L. EFFECTS OF FEEDBACK MODIFIERS ON THE STATE OF AGGREGATION OF HOMOSERINE DEHYDROGENASE OF RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1964 Jan;51:125–130. doi: 10.1073/pnas.51.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Gimmler H. Properties of the Isolated Intact Chloroplast at Cytoplasmic K Concentrations : I. Light-Induced Cation Uptake into Intact Chloroplasts is Driven by an Electrical Potential Difference. Plant Physiol. 1983 Sep;73(1):169–174. doi: 10.1104/pp.73.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicamelli C. A., Bryan J. K. Comparison of sensitive and desensitized forms of maize homoserine dehydrogenase. Plant Physiol. 1980 Feb;65(2):176–183. doi: 10.1104/pp.65.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Katinka M., Cossart P., Sibilli L., Saint-Girons I., Chalvignac M. A., Le Bras G., Cohen G. N., Yaniv M. Nucleotide sequence of the thrA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5730–5733. doi: 10.1073/pnas.77.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klungsoyr L., Hagemen J. H., Fall L., Atkinson D. E. Interaction between energy charge and product feedback in the regulation of biosynthetic enzymes. Aspartokinase, phosphoribosyladenosine triphosphate synthetase, and phosphoribosyl pyrophosphate synthetase. Biochemistry. 1968 Nov;7(11):4035–4040. doi: 10.1021/bi00851a034. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S., Bryan J. K. Characterization of ligand-induced states of maize homoserine dehydrogenase. Arch Biochem Biophys. 1983 Nov;227(1):210–224. doi: 10.1016/0003-9861(83)90364-8. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S., Bryan J. K. Ligand-induced interconversions of maize homoserine dehydrogenase among different states. Arch Biochem Biophys. 1983 Apr 15;222(2):449–463. doi: 10.1016/0003-9861(83)90544-1. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S., Bryan J. K. Use of monoclonal antibodies for the purification and characterization of the threonine-sensitive isozyme of maize homoserine dehydrogenase. Arch Biochem Biophys. 1986 Apr;246(1):250–262. doi: 10.1016/0003-9861(86)90471-6. [DOI] [PubMed] [Google Scholar]
- Matthews B. F., Farrar M. J., Gray A. C. Purification and Interconversion of Homoserine Dehydrogenase from Daucus carota Cell Suspension Cultures. Plant Physiol. 1989 Dec;91(4):1569–1574. doi: 10.1104/pp.91.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. F., Widholm J. M. Expression of aspartokinase, dihydrodipicolinic acid synthase and homoserine dehydrogenase during growth of carrot cell suspension cultures on lysine- and threonine-supplemented media. Z Naturforsch C. 1979 Dec;34(12):1177–1185. doi: 10.1515/znc-1979-1216. [DOI] [PubMed] [Google Scholar]
- Mills W. R. Photosynthetic formation of the aspartate family of amino acids in isolated chloroplasts. Plant Physiol. 1980 Jun;65(6):1166–1172. doi: 10.1104/pp.65.6.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Downton W. J. Potassium, sodium, and chloride content of isolated intact chloroplasts in relation to ionic compartmentation in leaves. Arch Biochem Biophys. 1984 Jan;228(1):197–206. doi: 10.1016/0003-9861(84)90061-4. [DOI] [PubMed] [Google Scholar]
- Zakin M. M., Duchange N., Ferrara P., Cohen G. N. Nucleotide sequence of the metL gene of Escherichia coli. Its product, the bifunctional aspartokinase ii-homoserine dehydrogenase II, and the bifunctional product of the thrA gene, aspartokinase I-homoserine dehydrogenase I, derive from a common ancestor. J Biol Chem. 1983 Mar 10;258(5):3028–3031. [PubMed] [Google Scholar]
- Zakin M. M., Garel J. R., Dautry-Varsat A., Cohen G. N., Boulot G. Detection of the homology among proteins by immunochemical cross-reactivity between denatured antigens. Application to the threonine and methionine regulated aspartokinases-homoserine dehydrogenases from Escherichia coli K 12. Biochemistry. 1978 Oct 3;17(20):4318–4323. doi: 10.1021/bi00613a032. [DOI] [PubMed] [Google Scholar]