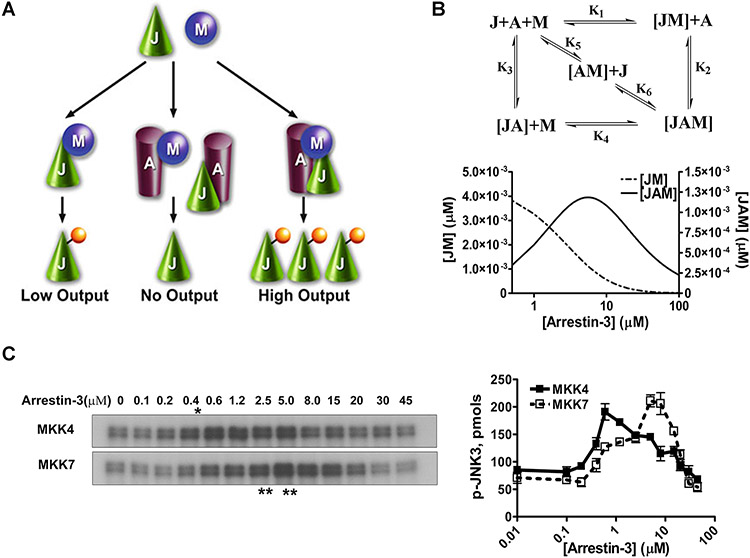
FIGURE 2.12.1.
Arrestin-3-mediated JNK3α2 activation by MKK4/7. (A) A three-state model showing the scaffolding mechanism of the two-kinase signaling module. A, J, and M designate arrestin-3, JNK3α2, and upstream kinases MKK4/7, respectively. Kinases can exist in three states: (a) interacting in solution, (b) bound to the scaffold to form incomplete complexes containing a single kinase, and (c) simultaneously assembled by arrestin-3 to form a complete signaling complex. (B) Six affinity constants (k1 through k6) describe the indicated binding equilibria. Calculated concentrations of JM (dotted line, left y axis) and JAM (solid line, right y axis) complexes at different arrestin-3 concentrations (KinTek Explorer 3.0; all six Kd values were set at 5 μM). (C) Representative autoradiograms showing JNK3α2 phosphorylated by MKK4 (upper panel) or MKK7 (lower panel) at the indicated concentration of arrestin-3 (10-sec incubation). The optimal arrestin-3 concentrations are indicated (*, for MKK4; **, for MKK7). (D) The effect of arrestin-3 concentration on JNK3α2 phosphorylation by both MKK4 and MKK7 is biphasic. The bands from the gels were excised and the radioactivity measured in a Tri-Carb liquid scintillation counter to quantify the incorporation of [32P]phosphate from [γ-32P]ATP into JNK3α2. Data from (Zhan et al., 2013b).