Abstract
Synapse loss strongly correlates with cognitive decline in Alzheimer’s disease (AD), but the underlying mechanisms are poorly understood. Deficient Wnt signaling contributes to synapse dysfunction and loss in AD. Consistently, a variant of the LRP6 receptor, (LRP6-Val), with reduced Wnt signaling, is linked to late-onset AD. However, the impact of LRP6-Val on the healthy and AD brain has not been examined. Knock-in mice, generated by gene editing, carrying this Lrp6 variant develop normally. However, neurons from Lrp6-val mice do not respond to Wnt7a, a ligand that promotes synaptic assembly through the Frizzled-5 receptor. Wnt7a stimulates the formation of the low-density lipoprotein receptor-related protein 6 (LRP6)–Frizzled-5 complex but not if LRP6-Val is present. Lrp6-val mice exhibit structural and functional synaptic defects that become pronounced with age. Lrp6-val mice present exacerbated synapse loss around plaques when crossed to the NL-G-F AD model. Our findings uncover a previously unidentified role for Lrp6-val in synapse vulnerability during aging and AD.
Lrp6-val induces synapse loss in aging and in Alzheimer’s disease by inhibiting the formation of the Wnt receptor complex.
INTRODUCTION
In Alzheimer’s disease (AD), memory impairment strongly correlates with synapse degeneration (1–3). Synaptic changes occur early in the disease, before amyloid-β (Aβ) plaque formation and neuronal loss (3, 4). At the later stages of the disease, further synapse loss is observed around Aβ plaques (5–8). It is well documented that the accumulation of oligomeric forms of Aβ triggers synapse dysfunction and degeneration (3, 4, 9). Numerous studies demonstrate that Aβ oligomers interfere with signaling pathways, which are critical for maintaining synapse integrity, resulting in synapse weakening and loss (10, 11). However, the molecular mechanisms that lead to synapse dysfunction and loss in AD remain poorly understood.
The canonical Wnt signaling pathway, required for synapse function and stability, is impaired in AD (Fig. 1A) (12–14). Wnt ligands and their receptors are expressed in many brain areas affected in AD (https://mouse.brain-map.org/). For instance, many Wnt ligands and Frizzled (Fz) receptors including Wnt7a, Wnt7b, and Fz5, and the Wnt co-receptor LRP6 are expressed in the postnatal and adult hippocampus (13, 15–18). However, it is unclear whether these ligands and receptors act in an autocrine or paracrine fashion in neurons. Wnt ligands, their receptors, and co-receptors play a critical role in synaptogenesis during postnatal development (18–21) and in synapse integrity in the adult brain (12, 13, 22, 23). The first piece of evidence that Wnt signaling is compromised in AD came from the finding that Dickkopf-1 (Dkk1), a secreted Wnt antagonist, is elevated in the brains of AD patients and AD mouse models (24, 25). Notably, Dkk1 is required for Aβ-induced synapse degeneration as blockade of Dkk1 protects against Aβ-mediated synapse loss (14, 26). Consistently, in vivo expression of Dkk1 in the adult brain induces synapse loss, long-term potentiation (LTP) defects (12), and memory impairment (12, 27), as observed in AD mouse models. Second, conditional knockout (cKO) of Lrp6 in an AD mouse model increases amyloid pathology and exacerbates cognitive deficits (13). Last, a genetic link between deficient Wnt signaling and AD came from the identification of three genetic variants of LRP6 associated with late-onset AD (LOAD) (28, 29). Notably, a nonsynonymous single-nucleotide polymorphism (SNP) (rs2302685) (Fig. 1B), which has an allele frequency of 0.17 in the European population (30), results in a conservative substitution of isoleucine to valine at amino acid 1062 (LRP6Ile-1062-Val; LRP6-Val herein). This substitution is located in the fourth β-propeller of the extracellular domain of LRP6 to which some Wnt ligands bind (Fig. 1B) (31–33). The LRP6-Val variant reduces Wnt signaling in cell lines in response to a Wnt ligand (28). However, the impact of this variant on brain development, neuronal connectivity, and amyloid pathology remains unexplored.
Fig. 1. Expression of LRP6-Val induces synaptic defects in cultured neurons.
(A) Diagram of canonical Wnt signaling. Left: In the absence of Wnt, β-catenin is sequestered and degraded by the destruction complex preventing transcription of Wnt target genes. Right: Wnt, LRP6, and Fz receptors form a complex. Activation of the pathway results in disheveled (Dvl) recruitment to the plasma membrane and disassembly of the destruction complex. β-Catenin accumulates and translocates to the nucleus enabling Wnt target gene transcription. (B) Schematic of LRP6 showing the location of the Lrp6-val SNP (red asterisk and arrow) and the areas to which the Wnt antagonist Dkk1 and Wnt ligands bind. (C) Confocal images of vGlut1 (red) puncta on isolated axons of neurons expressing enhanced green fluorescent protein (EGFP)–actin alone or EGFP-actin and human WT LRP6 or human LRP6-Val. Scale bar, 5 μm. (D) WT LRP6 promoted the assembly of presynaptic sites but LRP6-Val did not. N = 4 independent cultures, 10 to 12 axons per culture. Kruskal-Wallis with Dunn’s post hoc test. (E) Top: Confocal images of Homer1 (red) and GFP (green) of neurons expressing EGFP-actin and human WT LRP6 or human LRP6-Val. Scale bar, 21 μm. Bottom: Higher-magnification images show dendritic spines (GFP; green) and Homer1 (red) puncta along dendrites. Scale bar, 5 μm. (F) Left: Expression of LRP6-Val reduced spine density. Three independent cultures, 8 to 10 cells per culture. One-way analysis of variance (ANOVA) with Tukey’s post hoc test. Right: Expression of LRP6-Val failed to increase spine size. N = 3 independent cultures, 8 to 10 cells per culture. Kruskal-Wallis with Dunn’s post hoc. (G) LRP6-Val expression led to smaller and fewer Homer1 puncta. N = 3 independent cultures, 8 to 10 images per culture. One-way ANOVA with Tukey’s post hoc test. Data are represented as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001. ns, not significant.
Here, we investigated the impact of the Lrp6-val variant on the adult and aging hippocampus and in the pathogenesis of AD by generating a novel knock-in (KI) mouse model using CRISPR-Cas9 genome editing. Homozygous Lrp6-val mice developed normally but showed structural and functional synaptic defects in the hippocampus that became more pronounced with age. In these mice, we observed decreased levels of canonical Wnt signaling with age. Notably, neurons from Lrp6-val mice were unable to respond to Wnt7a or Wnt3a to promote synapse formation. As Wnt ligands promote the interaction between LRP6 and Fz (34), we examined whether the LRP6-Val variant affects this interaction by focusing on Fz5, a Wnt7a receptor required for presynaptic assembly in hippocampal neurons (15). We found that Wnt7a increased the association between wild-type (WT) LRP6 and Fz5, whereas this interaction was significantly impaired in the presence of LRP6-Val. Consistently, expression of LRP6-Val reduced Wnt signaling. Next, we examined the contribution of the Lrp6-val variant to AD pathogenesis by crossing these mice to hAPPNL-G-F/NL-G-F (NL-G-F), a KI AD mouse model. The Lrp6-val variant significantly increased synapse degeneration in NL-G-F mice. The valine substitution in the extracellular domain of LRP6 impairs its Wnt7a-mediated interaction with Fz5, affecting downstream signaling. These findings represent a significant advancement in the Wnt and AD fields by linking a genetic variant that affects Wnt signaling with the pathogenesis of the disease.
RESULTS
LRP6-Val fails to stimulate synaptic assembly and induces spine loss
LRP6 is required for synapse formation and maintenance (13, 20). Furthermore, the LRP6-Val variant is linked to LOAD (28), but its impact on neuronal connectivity has not been explored. To assess the effect of LRP6-Val on synapses, we expressed human WT LRP6 or human LRP6-Val in cultured hippocampal neurons. Expression of WT LRP6 increased the puncta number of vGlut1, a presynaptic marker, along axons (Fig. 1, C and D). In contrast, LRP6-Val failed to increase the number of presynaptic sites above control cells (Fig. 1, C and D). Thus, expression of the LRP6-Val variant is unable to induce presynaptic assembly in neurons.
We next examined whether the presynaptic assembly induced by expression of WT LRP6 was ligand dependent as the function of this Wnt co-receptor requires Wnts for signaling (35). To block the synaptogenic activity on Wnts in hippocampal neurons, we used the secreted Fz-related protein 1 (sFRP1) (19). The addition of sFRP1 blocked the increase in the puncta number of Bassoon, a presynaptic marker, along axons induced by expression of WT LRP6 (fig. S1). However, sFRP1 did not affect the phenotype of neurons expressing the LRP6-Val variant (fig. S1). These results indicate that endogenous Wnts are required for signaling through LRP6 resulting in presynaptic assembly.
We also examined the impact of LRP6-Val on dendritic spines (Fig. 1E). Expression of WT LRP6 increased the spine head width but had no effect on spine density (Fig. 1, E and F). In contrast, expression of LRP6-Val failed to increase the spine head width and decreased the spine density when compared to control and WT LRP6-expressing cells (Fig. 1, E and F). Thus, LRP6-Val is unable to promote dendritic spine growth while inducing spine loss. No differences in puncta number or volume of Homer1, a postsynaptic marker, were found between control and WT LRP6-expressing neurons (Fig. 1, E and G). In contrast, consistent with the reduced number of spines, fewer Homer1 puncta and a decrease in puncta volume were observed in LRP6-Val–expressing neurons compared to control neurons (Fig. 1, E and G). Thus, expression of LRP6-Val in neurons decreases the formation of both pre- and postsynaptic sites compared to WT LRP6.
Lrp6-val KI mice develop normally, and LRP6-Val does not affect its synaptic localization
To investigate the in vivo role of the Lrp6-val variant, we generated a novel KI mouse model using CRISPR-Cas9 genome editing. The Lrp6 A→G point mutation, which results in the substitution of isoleucine for valine, was introduced at the endogenous Lrp6 locus in the mouse genome, creating a mouse line that carries the Lrp6-val variant globally. DNA sequencing confirmed the successful generation of both heterozygous (Lrp6-val het) and homozygous (Lrp6-val hom) KI animals (Fig. 2A). Lrp6-val hom mice developed normally with no visible morphological abnormalities or changes in weight (fig. S2, A and B). Furthermore, Lrp6-val het and Lrp6-val hom mice exhibited similar synaptic phenotypes (see below). Here, we primarily focused our attention on Lrp6-val hom (Lrp6-val) mice.
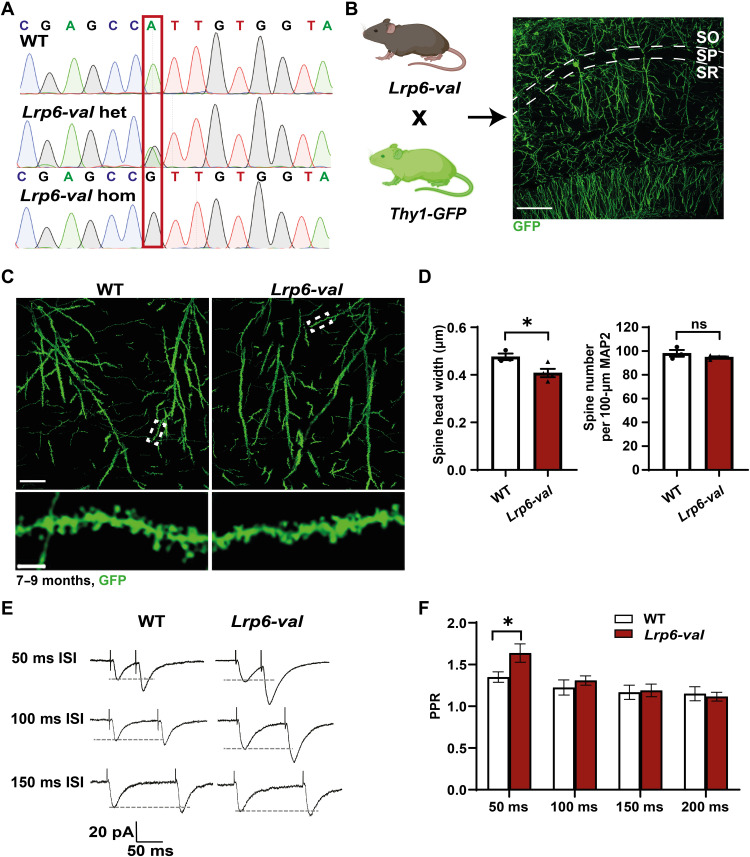
Fig. 2. Lrp6-val mice exhibit synaptic defects at 7 to 9 months.
(A) Sanger trace examples of WT, Lrp6-val heterozygous (Lrp6-val het), and Lrp6-val homozygous (Lrp6-val hom) KI mice. (B) Dendritic spines were analyzed in Lrp6-val hom KI mice crossed to a Thy1-GFP line. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. Scale bar, 100 μm. (C) Confocal images of apical dendrites of CA1 pyramidal neurons of WT and Lrp6-val mice at 7 to 9 months. Scale bar, 25 μm. Insets show spines along a dendrite. Scale bar, 3 μm. (D) Lrp6-val mice display reduced spine head width. WT, N = 3; Lrp6-val N = 4. Unpaired t test. *P < 0.05. (E) Representative paired-pulse recordings of synaptic currents from WT and Lrp6-val brain slices at different ISIs. (F) Graph displays the mean PPR from all recorded cells. Lrp6-val increased the PPR at 50-ms ISIs. N = 11 to 21 cells recorded from four to five animals per genotype. Repeated measures one-way ANOVA with Tukey’s post hoc test. *P < 0.05. Data are represented as means ± SEM.
We next investigated whether the Lrp6-val variant was differentially expressed or affected its synaptic localization. The mRNA and protein levels of LRP6 in the hippocampus of Lrp6-val mice were unchanged (fig. S2, C to E). To assess its synaptic localization, we used structured illumination microscopy (SIM). Both WT LRP6 and LRP6-Val were present at approximately 80% of excitatory synapses (fig. S2, F and G), but no differences in LRP6 localization were observed between neurons from WT and Lrp6-val mice (fig. S2G). Thus, carrying the Lrp6-val variant does not alter the levels or the synaptic localization of this co-receptor.
Lrp6-val causes progressive synaptic defects with age
Spine formation and growth are modulated by Wnt signaling in the hippocampus (18, 19, 23, 36). Given that the expression of LRP6-Val failed to stimulate spine growth and induced spine loss in cultured neurons (Fig. 1, E and F), we examined the in vivo impact of Lrp6-val on these postsynaptic structures. We analyzed the dendritic spines on the apical dendrites of Cornu Ammonis-1 (CA1) pyramidal neurons in adult Lrp6-val KI mice crossed to a Thy1-GFP expressing line (Fig. 2B) (37). Although no differences in the spine density were observed, the spine head width was reduced in Lrp6-val mice compared to WT mice at 7 to 9 months (Fig. 2, C and D). Thus, adult mice carrying the Lrp6-val variant display impaired spine growth.
Given our data on the synaptic localization of LRP6 and the role of Wnt signaling in synaptic function (38, 39), we examined synaptic transmission in the Lrp6-val mice at Schaffer collateral (SC)–CA1 synapses, where deficient Wnt signaling leads to defects in synaptic transmission (12). Evoked excitatory postsynaptic currents (EPSCs), in response to SC stimulation of increasing intensity [input-output (I/O) curve], were recorded in CA1 pyramidal cells at 7 to 9 months. No significant differences were observed between WT and Lrp6-val mice even at high-stimulation intensities (fig. S3, A and B), suggesting that basal synaptic transmission at SC-CA1 synapses is unaffected by the presence of the Lrp6-val variant at this age.
Wnt signaling–deficient mice exhibit reduced neurotransmitter release probability (22, 40). We therefore investigated the possible defects in neurotransmitter release. EPSCs evoked at brief intervals at SC-CA1 synapses were recorded from WT and Lrp6-val mice at 7 to 9 months. We analyzed the paired-pulse ratio (PPR), which depends on presynaptic short-term plasticity mechanisms and is inversely correlated with release probability (41, 42). Lrp6-val mice exhibited increased PPRs compared to WT mice at 50-ms interstimulus intervals (ISIs), consistent with a reduced release probability at 7 to 9 months (Fig. 2, E and F). Thus, neurotransmitter release is compromised in adult Lrp6-val mice.
Given that cKO mice for Lrp6 exhibit age-dependent synaptic deficits (13), we interrogated whether synaptic defects become more pronounced with age. We evaluated dendritic spines in older Lrp6-val;Thy1-GFP mice (Fig. 3A). Our analyses revealed a significant decrease in both spine density and head width in Lrp6-val mice compared to WT mice at 12 to 14 months (Fig. 3B). As the spine size but not spine number was affected in 7- to 9-month-old Lrp6-val mice, these results demonstrate that spine deficits become more severe with age.
Fig. 3. Lrp6-val mice display postsynaptic defects and impaired basal synaptic transmission, synaptic vesicle release, and RRP size at 12 months.
(A) Top: Dendritic spines were analyzed at 12 to 14 months in Lrp6-val KI mice crossed to a Thy1-GFP line. Scale bar, 25 μm. Bottom: Confocal images of regions of interest containing apical dendrites of CA1 pyramidal neurons in WT and Lrp6-val mice. Scale bar, 3 μm. (B) Lrp6-val mice have smaller and fewer spines. WT, N = 4; Lrp6-val, N = 4. Unpaired t test. (C) Representative traces of EPSCs elicited at increasing stimulation voltages with an average of three responses for each stimulus voltage. (D) I/O curves showing a significant reduction in EPSC amplitude in hippocampal slices from Lrp6-val mice. N = 12 to 13 cells from four animals per genotype. Repeated measure one-way ANOVA with Tukey’s post hoc test. (E) Representative traces of paired pulse evoked EPSCs at different ISIs using brain slices from WT and Lrp6-val mice. (F) Graph displays the mean PPR from all cells. Lrp6-val mice display increased PPR at 50- and 100-ms ISI. N = 13 to 14 cells from four animals per genotype. Repeated measure one-way ANOVA with Tukey’s post hoc test. (G) Representative traces of EPSCs elicited by a 20-Hz electrical stimulation for 3 s recorded from WT and Lrp6-val mice. (H) Graph showing reduced mean cumulative charge in Lrp6-val mice. N = 12 cells from four animals per genotype. Repeated measure one-way ANOVA with Tukey’s post hoc test. (I) Graph displays the RRP size, obtained from all cells. Lrp6-val mice exhibited a reduced RRP. N = 12 cells from four animals per genotype. Unpaired Student’s t test. Data are represented as means ± SEM. *P < 0.05 and ***P < 0.001.
Next, we evaluated basal synaptic transmission in 12-month-old mice by performing I/O curve recordings in hippocampal slices. The amplitude of evoked EPSCs was significantly smaller in 12-month-old Lrp6-val mice compared to WT mice at all stimulus intensities (Fig. 3, C and D). Thus, the Lrp6-val variant impairs basal synaptic transmission at this age but not at 7 to 9 months (fig. S3, A and B). We then measured neurotransmitter release probability and found that the PPR was significantly higher in Lrp6-val mice compared to WT mice at both 50- and 100-ms ISI, consistent with a reduction in release probability (Fig. 3, E and F). Given that PPR was significantly higher at 50-ms but not at 100-ms ISI in Lrp6-val mice at 7 to 9 months, these results suggest that defects in neurotransmitter release are more pronounced in older animals.
To further define the role of Lrp6-val in neurotransmitter release at 12 months, we recorded responses to a 3-s high-frequency stimulus train (20 Hz), which fully depletes presynaptic terminals of the readily releasable pool (RRP) (43). Using the first-order correction for vesicle recycling (43), a significant reduction in the size of the RRP was observed in 12-month-old Lrp6-val mice when compared to WT mice (Fig. 3, G to I). However, synaptic vesicle fusion efficiency and recycling rate were unaffected (fig. S3C), suggesting that the defect in release probability is due to a reduced RRP.
The defects in vesicular release probability at 12 months suggested potential structural changes at presynaptic terminals of Lrp6-val mice. To investigate this hypothesis, we evaluated the number and size of presynaptic boutons using the presynaptic marker, vGlut1, in the CA1 stratum radiatum (SR) of Lrp6-val mice at 12 to 14 months (Fig. 4A). Lrp6-val mice had smaller and fewer vGlut1 puncta compared to WT mice (Fig. 4B). As Lrp6-val mice exhibited deficits in synaptic vesicle release, due to a smaller RRP (Fig. 3), we assessed possible ultrastructural changes by electron microscopy (EM). We observed fewer synaptic vesicles at presynaptic terminals but no differences in the length of the postsynaptic density (PSD) at SC-CA1 synapses of Lrp6-val mice (Fig. 4, C and D). These findings indicate that impaired neurotransmitter release in Lrp6-val mice is due to a reduction in synaptic vesicle number, consistent with a reduced RRP.
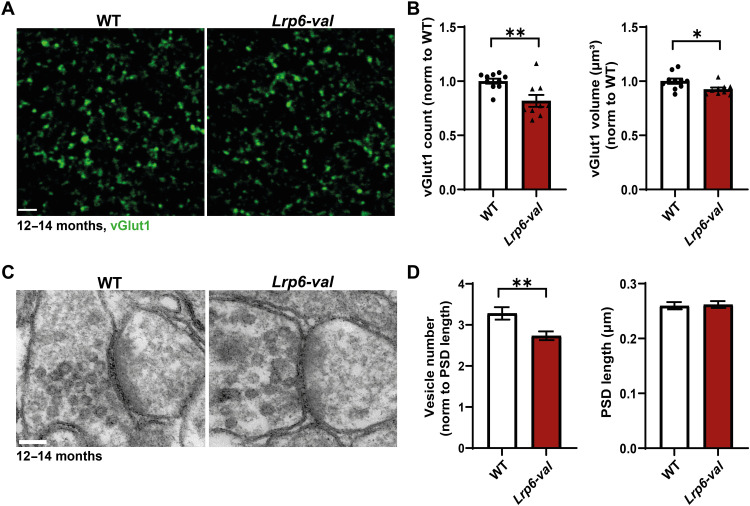
Fig. 4. Presynaptic defects of Lrp6-val mice at 12 to 14 months.
(A) Confocal images of vGlut1-labeled excitatory presynaptic terminals in the CA1 SR area of WT and Lrp6-val mice at 12 to 14 months. Scale bar, 2 μm. (B) Lrp6-val mice had fewer and smaller vGlut1 puncta. WT, N = 10; Lrp6-val, N = 9. Unpaired t test. *P < 0.05 and **P < 0.01. (C) EM images of an excitatory synapse of 12- to 14-month-old WT and Lrp6-val mice. Scale bar, 100 nm. (D) Lrp6-val mice had fewer synaptic vesicles, but no changes in PSD length were observed. N = 5, 19 to 25 images per animal. Mann-Whitney test. **P < 0.01. Data are represented as means ± SEM.
Given that defects at the pre- and postsynaptic sites were exacerbated with age in Lrp6-val mice, we compared the number of excitatory synapses at the different ages. Synapses were quantified on the basis of the colocalization of Bassoon and Homer1, pre- and postsynaptic markers, respectively, in the CA1 SR region. At 7 to 9 months and 12 months, no differences were observed between WT and Lrp6-val mice (Fig. 5, A to D). However, a significant reduction in the number of excitatory synapses was detected at 16 to 18 months in Lrp6-val mice when compared to WT mice (Fig. 5, E and F). Because of the age of these animals, we examined possible neuronal loss, which could affect synapse number, in the stratum pyramidale layer using 4′,6-diamidino-2-phenylindole (DAPI) and NeuN (fig. S4A). No difference in the percentage of NeuN-positive cells was identified between WT and Lrp6-val mice at 16 to 18 months (fig. S4B). Thus, the Lrp6-val variant confers increased synaptic vulnerability as animals age, a process that is not due to changes in neuronal number.
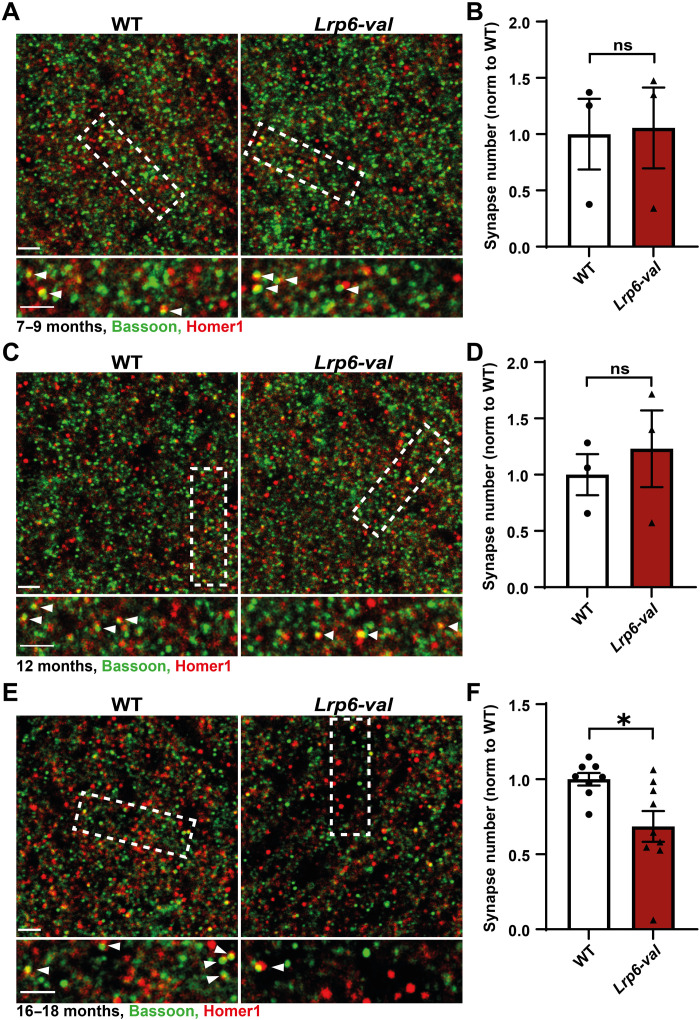
Fig. 5. Lrp6-val mice exhibit synapse loss with age.
(A, C, and E) Confocal images of the CA1 SR of WT and Lrp6-val mice labeled with Bassoon (green) and Homer1 (red) at 7 to 9 months (A), 12 months (C), and 16 to 18 months (E). Scale bars, 2.5 μm. Insets display high-magnification images of synapses. Scale bars, 2 μm. (B and D) Quantification of synapse number, based on the colocalization of pre- and postsynaptic puncta, showed no differences between WT and Lrp6-val mice at 7 to 9 months (B) or 12 months (D). N = 3 per genotype. Unpaired t test. (F) Synapse number was significantly reduced in Lrp6-val mice 16 to 18 months. WT, N = 8; Lrp6-val, N = 9. Unpaired t test. *P < 0.05. Data are represented as means ± SEM.
As the Lrp6-val variant is predominantly present as heterozygous in the human population (30), we also analyzed heterozygous Lrp6-val mice at 12 to 14 months. Although no differences were detected in the presynaptic marker vGlut1 between WT and heterozygous mice, homozygous mice exhibited a reduction in vGlut1 puncta number (fig. S5A). Postsynaptically, both heterozygous and homozygous Lrp6-val mice exhibited a decrease in the number and size of dendritic spines when compared to WT mice (fig. S5B). Thus, carrying one allele of the Lrp6-val confers postsynaptic vulnerability.
Wnt signaling is impaired in Lrp6-val mice, and Lrp6-val neurons do not respond to Wnt7a
To start addressing the molecular mechanisms through which aged Lrp6-val mice exhibit synaptic defects, we investigated whether canonical Wnt signaling was affected in aged mice. We therefore evaluated the mRNA levels of Axin2, a target of canonical Wnt signaling (fig. S6A) (44). Axin2 expression was not affected in 4- to 7-month-old Lrp6-val mice (fig. S6B), but it was significantly decreased in 12- to 15-month-old Lrp6-val mice compared to WT (fig. S6C). Thus, Wnt signaling is compromised with age in Lrp6-val mice, consistent with the appearance of synapse defects.
The above findings led us to hypothesize that the LRP6-Val receptor does not signal properly. We therefore interrogated whether neurons from Lrp6-val mice responded to exogenous Wnts (Fig. 6A). Previous studies demonstrate that Wnt7a promotes the formation of excitatory synapses in hippocampal neurons (15–18). Consistently, Wnt7a significantly increased the number of synapses in neurons from WT mice compared to neurons exposed to a control vehicle (Fig. 6, B and C). However, Wnt7a was unable to increase synapse number in hippocampal neurons from Lrp6-val mice (Fig. 6B and C). Crucially, the same effect was observed with a second concentration of Wnt7a (fig. S7, A and B). We also examined whether neurons from Lrp6-val mice respond to Wnt3a, another ligand involved in presynaptic assembly (16, 45). Moreover, the Ile to Val amino acid substitution in LRP6 is in the Wnt3a binding site (31–33), and expression of LRP6-Val in a cell line reduces Wnt3a-mediated signaling (28). We found that Wnt3a increased the number of synapses in WT neurons but failed to increase synapse number in Lrp6-val neurons (fig. S7, A and C). Thus, Lrp6-val neurons respond to neither Wnt7a nor Wnt3a. The lack of response to Wnt ligands was not due to decreased levels of the LRP6-Val receptor at the plasma membrane, as the surface levels of LRP6 were the same between WT and Lrp6-val neurons (fig. S7, A, D, and E). Thus, Lrp6-val impairs the ability of neurons to respond to exogenous Wnt ligands without affecting its surface localization.
Fig. 6. Neurons from Lrp6-val mice fail to respond to Wnt7a and the presence of LRP6-Val affects the formation of the Wnt receptor complex and downstream signaling.
(A) Diagram depicting hippocampal neuron isolation from WT and Lrp6-val mice. (B) Images of WT and Lrp6-val neurons treated with recombinant Wnt7a. vGlut1 (green), Homer1 (red), and MAP2 (Blue). Scale bar, 5 μm. (C) Wnt7a (200 ng/ml) increased synapse number in WT neurons but not in Lrp6-val neurons. N = 4 independent cultures. Two-way ANOVA with Games-Howell post hoc test. (D) Schematic of proximity ligation assay (PLA) to detect LRP6 and Fz5-HA interaction in close proximity (<40 nm). (E) Confocal images of HeLa cells expressing GFP (control), Fz5-HA, and WT LRP6 or LRP6-Val treated with control vehicle (Veh) or Wnt7a. GFP, green; PLA, red; and DAPI, blue. Scale bar, 10 μm. (F) The PLA signal intensity per cell was increased in cells expressing Fz5-HA and WT LRP6 or LRP6-Val compared to cells expressing GFP. N = 3 independent experiments. One-way ANOVA with Tukey’s post hoc test. (G) Wnt7a increased the PLA signal in cells expressing WT LRP6 and Fz5-HA but not in cells expressing LRP6-Val and Fz5-HA. N = 3 independent experiments. Two-way ANOVA with Tukey’s post hoc test. (H) Wnt7a increased pLRP6 when normalized to total LRP6 in HeLa cells expressing WT LRP6 and Fz5-HA but not in cells expressing LRP6-Val and Fz5-HA. WT LRP6 + Fz5-HA Veh, N = 50 cells; WT LRP6 + Fz5-HA Wnt7a, N = 53 cells; LRP6-Val + Fz5-HA Veh, N = 71 cells; and LRP6-Val + Fz5-HA Wnt7a, N = 61 cells from three independent experiments. Kruskal-Wallis with Dunn’s post hoc test. Data are represented as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001. A.U. arbitrary units.
LRP6-Val impairs the formation of the Wnt7a-induced LRP6-Fz5 complex and downstream signaling
The lack of response to Wnt7a and Wnt3a in neurons from Lrp6-val mice suggested that the presence of LRP6-Val could impair the formation of a complex between LRP6 and Fz receptors, which is crucial for the activation of the canonical Wnt signaling pathway (34). We specifically chose to examine the interaction between Fz5 and LRP6 because this Fz receptor is required for Wnt7a-mediated presynaptic assembly in hippocampal neurons (15). We coexpressed Fz5-HA with WT LRP6 or LRP6-Val in HeLa cells to evaluate their interaction, which is induced by Wnt7a, using proximity ligation assay (PLA), a technique that allows the detection of protein-protein interactions at less than 40 nm in cells (Fig. 6D) (46, 47). The PLA signal was elevated in cells expressing Fz5-HA and WT LRP6 or LRP6-Val when compared to control green fluorescent protein (GFP)–expressing cells (Fig. 6, E and F). No differences in the interaction were observed between cells expressing Fz5-HA and WT LRP6 or LRP6-Val under basal conditions (Fig. 6, E to G). In contrast, Wnt7a significantly increased the PLA signal intensity in cells expressing Fz5-HA and WT LRP6 but not in cells expressing Fz5-HA and LRP6-Val (Fig. 6, E and G). These results were not due to changes in the surface levels of these receptors, as determined by surface biotinylation (fig. S8, A to E). Together, these results demonstrate that the presence of LRP6-Val impairs the formation of the Wnt-induced LRP6-Fz receptor complex, which is required for signaling.
We next examined whether downstream signaling was affected by the presence of LRP6-Val. The formation of the LRP6-Fz complex promotes the intracellular phosphorylation of LRP6 at multiple sites, including at serine-1490, which is important for LRP6 function (48). We therefore tested whether this posttranslational modification was affected by the LRP6-Val variant. Cells expressing WT LRP6 and Fz5-HA or LRP6-Val and Fz5-HA were exposed to recombinant Wnt7a, and the level of phosphorylated LRP6 (pLRP6) at serine-1490 was evaluated by immunofluorescence microscopy. As expected, Wnt7a significantly increased the level of pLRP6 in cells expressing WT LRP6 (Fig. 6H and fig. S8F). However, no changes were observed in the pLRP6 levels in cells expressing the LRP6-Val variant (Fig. 6H and fig. S8F). Thus, the presence of LRP6-Val affects the formation of the LRP6-Fz5 complex and subsequent downstream signaling.
Lrp6-val does not affect plaque load in NL-G-F mice
The findings that the LRP6-Val variant is associated with LOAD (28) and that cKO of Lrp6 in neurons of the APP/PS1 AD mouse model exacerbates the formation of Aβ plaques (13) led us to interrogate the impact of the Lrp6-val variant on amyloid pathology. We crossed the Lrp6-val mice to the NL-G-F, a KI AD mouse model that carries a humanized Aβ region of APP with three mutations associated with AD (49). In NL-G-F mice, plaque deposition begins around 2 months, with a significant increase in the number at 7 months (49). We assessed the impact of Lrp6-val on plaque load in homozygous NL-G-F mice. No differences in plaque burden were detected between NL-G-F and NL-G-F;Lrp6-val mice at 2, 7, and 10 months (fig. S9, A to F). Furthermore, Aβ coverage (area covered by Aβ) was unaltered in NL-G-F;Lrp6-val mice compared to NL-G-F mice at 7 and 10 months (fig. S9, C to F). Thus, the presence of the Lrp6-val variant does not exacerbate plaque load in NL-G-F mice at the ages examined.
Next, we examined the levels of soluble and insoluble Aβ42, as this peptide is the most abundant Aβ species in NL-G-F mice (49). However, no differences in the level of Aβ42 was observed between NL-G-F and NL-G-F;Lrp6-val mice at 10 months (fig. S10). These results are consistent with our findings that Aβ plaque number and Aβ coverage are unaffected in NL-G-F mice when carrying the Lrp6-val variant.
Lrp6-val exacerbates synapse loss in NL-G-F mice
Although the impact of Lrp6 cKO on Aβ plaque load has been examined in the context of AD (13), changes in synapse density were not investigated. Our findings that Lrp6-val mice exhibit synaptic defects led us to interrogate the contribution of this SNP to synapse vulnerability in AD. We therefore evaluated the impact of Lrp6-val on synapses in NL-G-F mice at 2 months of age, when plaques begin to form (49). However, no differences in synapse number were observed between WT, Lrp6-val, NL-G-F, and NL-G-F;Lrp6-val mice at this early stage (fig. S11, A and B).
We next investigated the impact of the Lrp6-val variant on synapses at 7 months of age in NL-G-F mice, by which time a significant number of plaques are present (49). Given that synapse loss is particularly pronounced around Aβ plaques (Fig. 7A) (5–8), synapse number was quantified at increasing distances from the center of a plaque or from a similar area in WT and Lrp6-val mice, in the CA1 SR (Fig. 7B). At 0 to 10 μm from the center of a plaque, a significant reduction in synapse number was observed in NL-G-F mice when compared to WT mice or to Lrp6-val mice (Fig. 7, B and C). A further decrease in synapse number was observed between NL-G-F;Lrp6-val double-mutant mice when compared to NL-G-F, Lrp6-val, or WT mice. This effect was also observed at further distances from the center of a plaque (Fig. 7, B and C). Thus, carrying the Lrp6-val variant exacerbates synapse loss around plaques in NL-G-F mice.
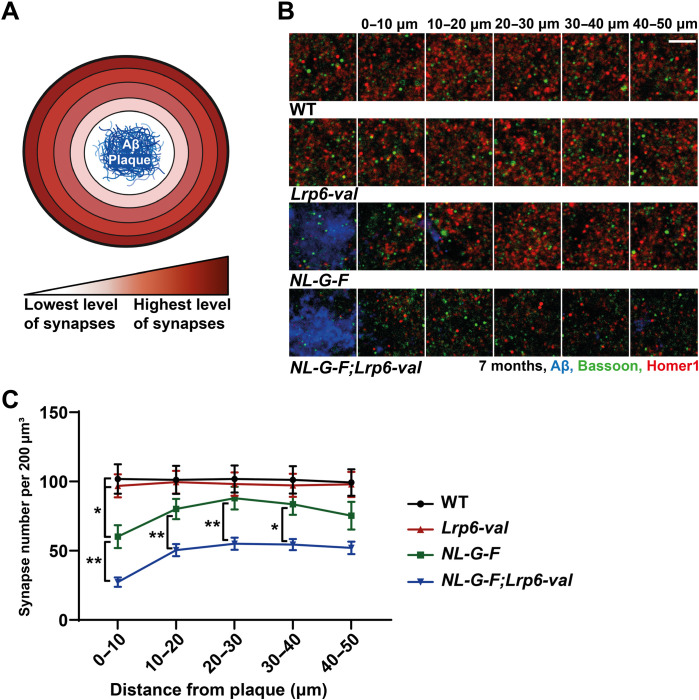
Fig. 7. Lrp6-val exacerbates synapse loss around plaques in NL-G-F mice at 7 months.
(A) Diagram shows synapse loss around an Aβ plaque (blue). (B) Confocal images of Bassoon (green) and Homer1 (red) at increasing distances from the center of an Aβ plaque (blue) in NL-G-F and NL-G-F;Lrp6-val mice or an equivalent point in WT and Lrp6-val mice, in the CA1 SR at 7 months. Scale bar, 4 μm. (C) NL-G-F mice displayed fewer synapses compared to WT mice or to Lrp6-val mice at 0 to 10 μm from the center of a plaque. Synapse number was reduced in NL-G-F;Lrp6-valmiceat 0 to 40 μm from the center of a plaque compared to NL-G-F mice. A significant reduction in synapse number was detected in NL-G-F;Lrp6-val micewhen compared to WT mice or Lrp6-val mice at all distances from the center of a plaque. WT = 17 slices from six animals, Lrp6-val = 18 slices from six animals, NL-G-F = 18 slices from seven animals, and NL-G-F;Lrp6-val = 19 slices from seven animals. Repeated measure two-way ANOVA with Tukey’s post hoc test. *P < 0.05 and **P < 0.01. Data are represented as means ± SEM.
To determine whether the enhanced synapse degeneration at 7 months was due to deregulation of Wnt signaling components, we examined the expression of key Wnt ligands and receptors by quantitative polymerase chain reaction (qPCR) in the hippocampi of WT, Lrp6-val, NL-G-F, and NL-G-F;Lrp6-val mice. However, no differences were detected in the expression of the Wnt receptors Lrp6 and Fz5 or the Wnt ligands Wnt7a and Wnt7b (fig. S12).
We also examined whether synapse number was more pronounced in the double-mutant mice when compared to NL-G-F mice at 10 months as Aβ pathology becomes more severe with age in NL-G-F mice (49). We found no significant differences in synapse loss around plaques between NL-G-F and NL-G-F; Lrp6-val mice at this age (fig. S11, C and D). Together, our results demonstrate that carrying the LRP6-Val receptor accelerates synapse loss in NL-G-F mice when synaptic defects become evident but not with progressive pathogenesis.
DISCUSSION
Here, we evaluate the impact of the LRP6-Val variant, which is linked to LOAD, on synaptic connectivity during aging and in AD. We generated a novel KI mouse model carrying this variant. Analyses of homozygous Lrp6-val mice reveal that this SNP induces age-associated defects in synaptic transmission and neurotransmitter release. Lrp6-val mice also exhibit progressive structural synaptic defects in the hippocampus. The presence of Lrp6-val exacerbates synapse loss around plaques in the NL-G-F AD model. Investigation into the molecular mechanisms underlying the synaptic defects elicited by LRP6-Val reveals a defect in the formation of the LRP6-Fz5 complex mediated by Wnt7a, which explains the decreased downstream signaling that is only evident with age. Thus, our studies also reveal a previously unrecognized molecular mechanism by which this variant affects Wnt signaling and uncover a role for LRP6-Val in synapse degeneration in AD.
The LRP6 receptor, which localizes to synapses, promotes the assembly of synapses. Using superresolution microscopy, we found that LRP6 is present at both pre- and postsynaptic sites. Expression of WT LRP6 in neurons increases the number of presynaptic puncta and promotes spine growth, consistent with activation of the Wnt pathway in neurons (16, 18) and in agreement with a previous study showing that LRP6 localizes to both pre- and postsynaptic sites and is required for synaptogenesis in cultured neurons (20). In contrast, expression of the LRP6-Val variant in neurons neither increases the number of presynaptic sites nor affects spine size but induces spine loss. Together, these findings demonstrate that expression of the LRP6-Val variant in hippocampal neurons fails to promote synapse formation.
Homozygous Lrp6-val mice exhibit structural and functional synaptic defects that become more pronounced with age. At 7 to 9 months, we observe reduced spine head width without changes in spine number or synapse number, whereas at 12 to 14 months both spine number and head width and the number of vGlut1 puncta are decreased. Moreover, defects in basal synaptic transmission and synaptic vesicle release, due to a smaller RRP, are exacerbated from 7 to 9 months to 12 to 14 months in homozygous Lrp6-val mice. However, no changes in synapse number are observed at these ages. In contrast, homozygous Lrp6-val mice exhibit a reduced number of excitatory synapses at 16 to 18 months. Heterozygous Lrp6-val mice also exhibit synaptic defects, suggesting that carrying a single allele of this variant confers synaptic vulnerability. Thus, the presence of the LRP6-Val variant contributes to progressive synaptic dysfunction and synapse degeneration.
Multiple variants of LRP6 have been linked to various age-associated diseases (28, 50, 51), including the LRP6-Val variant studied in this work, which has an allele frequency of 0.17 in the European population (30). First, LRP6-Val is associated with a 60% increase in bone fracture risk in older men (50). Second, LRP6-Val is a risk factor for carotid artery atherosclerosis in hypertensive patients over the age of 65 (51). Last, LRP6-Val is associated with LOAD (28). Thus, carrying the LRP6-Val variant results in age-related phenotypes that extend beyond the nervous system.
What are the molecular mechanisms that contribute to synaptic defects in the Lrp6-val mice? The synaptic deficits of Lrp6-val mice are not due to defects in the levels or localization of the LRP6-Val protein, as similar protein levels are found at synapses and the cell surface when compared to wildtype LRP6. This finding suggests possible defects in downstream signaling. Consistent with this hypothesis, Wnt7a and Wnt3a fail to induce excitatory synapse formation in neurons isolated from Lrp6-val mice. This lack of response correlates with defects in the interaction between Fz5 and LRP6-Val in response to Wnt7a and reduced downstream Wnt signaling as determined by the levels of pLRP6 and the levels of Axin2 expression at 12 to 15 months in Lrp6-val mice. Moreover, expression of LRP6-Val attenuates canonical Wnt signaling as evaluated by the TOPFlash assay (28). Thus, the presence of LRP6-Val impairs the formation of the Wnt receptor complex in response to Wnt7a, which decreases downstream signaling resulting in synaptic dysfunction and synapse degeneration.
The lack of response of Lrp6-val neurons to exogenous Wnt7a or Wnt3a suggests that LRP6-Val could act like a null mutant. However, Lrp6-val mice do not display developmental defects as observed in Lrp6 full KO mice, which die at birth due to severe embryonic defects (52). Thus, the Lrp6-val exhibits a hypomorphic phenotype. The lack of an early embryonic phenotype in the Lrp6-val mice could be due to the differential expression of different auxiliary proteins for the LRP6 co-receptor at different ages. These auxiliary proteins could compensate for signaling defects in the Lrp6-val mice during development but not in the adult brain.
The synaptic defects are manifested with age in the Lrp6-val mice. A possible explanation for this phenotype is that canonical Wnt signaling is dampened with age. Canonical Wnt signaling and several Wnt ligands, including Wnt7a, are reduced in the aging brain (53, 54). On the basis of these findings, we propose that reduced levels of Wnt proteins with age combined with the presence of a less functional receptor, such as LRP6-Val, contributes to the manifestation of age-dependent synapse loss in Lrp6-val mice.
In the context of AD, our studies demonstrate that carrying the Lrp6-val variant increases synapse vulnerability. Lrp6-val exacerbates synapse loss surrounding plaques in the NL-G-F model at 7 months. This is not due to differential expression of Wnt components, increased Aβ42 levels, or plaque load. Our findings are in contrast with those reported using the cKO of Lrp6 crossed to the APP/PS1 AD model, which exhibit increased Aβ40 and Aβ42 levels and enhanced plaque load (13). These differing results could be due to various reasons. First, our KI model contains a single–amino acid substitution in the Lrp6 gene, which is likely to result in a milder phenotype than that observed in the cKO model. Second, the cKO of Lrp6 was studied in APP/PS1, a transgenic model that overexpresses mutant APP and Presenilin1 (13). In contrast, the NL-G-F is a KI model with normal levels of APP. Thus, the differences between the findings presented here and the previous study could be explained by the models analyzed.
Understanding how risk factors contribute to the pathogenesis of AD is critical for identifying therapeutic targets to prevent or ameliorate synaptic dysfunction and synapse loss in this condition. Our findings uncover the impact of a genetic variant of LRP6 associated with LOAD on synapse vulnerability with age and in the context of AD. Thus, these findings further strengthen the link between deficient Wnt signaling and synapse loss in the aging and AD brain.
MATERIALS AND METHODS
Animals
Experiments with mice were carried out under personal and project licenses granted by the U.K. Home Office in accordance with the Animals (Scientific Procedures) Act 1986 and were approved by the University College London ethical committee. Animals were housed in ventilated cages with a 12-hour light/12-hour dark cycle and ad libitum access to food and water. Both male and female animals were used. Ages are specified in the figure legends.
Generation of Lrp6-val mutant mice
Single-strand oligonucleotides (ssODNs) were synthesized by Integrated DNA Technologies (IDT) (ssODN, CATCAGAGGCAGTCTCAGGCTGTGGCTTTGGAACATACCCTTTCTCGGGGTTTACCACAACGGCTCAGGTCTGTCTTGCTCGCCTTTTAGAACCACTCCAACTGATCGTCCATCTAATC). ssODNs were positioned adjacent to the guide RNA (gRNA) site: ACAGACCTCGAGCCATTGTGG. gRNA oligonucleotides were synthesized, and the two strands were annealed and cloned using Bsa I into a vector containing the gRNA backbone and a T7 promoter for RNA production. For Cas9 mRNA production, the vector from (55) was modified to contain the T7 promoter. Four- to 5-week-old C57BL/6NTac females were superovulated by injection of 5 IU of pregnant mare’s serum, and 48 hours later, 5 IU of human chorionic gonadotrophin (HCG) was injected. Females were mated with C57BL/6NTac males. Cumulus oocyte complexes were dissected from oviducts 21 to 22 hours after HCG and treated with hyaluronidase. Fertilized one-cell embryos were maintained at 37°C in KSOM media before cytoplasmic injection. Twenty-four to 27 hours after HCG, Cas9 mRNA (50 ng/μl), gRNA (25 ng/μl) (each), and oligonucleotide (100 ng/μl) were injected into the cytoplasm of fertilized one-cell embryos held in FHM medium. Viable embryos were transferred on the same day by oviducal embryo transfer into 0.5-day postcoital pseudo-pregnant female F1 (CBA/C57BL/6J) recipients. Homozygous Lrp6-Val C57BL/6NTac mice were backcrossed to C57BL/6J mice.
Genotyping
Lrp6-val mice were crossed to the Thy1-GFP mice or NL-G-F model to obtain Lrp6-val;Thy1-GFP mice and NL-G-F;Lrp6-val mice, respectively. Genotyping was performed on ear biopsies using the following primers: Lrp6 WT (forward: GATACGTTGCTTTAATGCCTTTAGCAAGACAGACCTCGAGCAA), Lrp6-val (forward: TGGCGGCAAGACAGACCTCGAGCAG), Lrp6 (WT and Val) (reverse: AACGCGCAACGAAGGGTGAGGAGGCATCA), NL-G-F (5′-ATCTCGGAAGTGAAGATG-3′, 5′-ATCTCGGAAGTGAATCTA-3′, 5′-TGTAGATGAGAACTTAAC-3′, and 5′-CGTATAATGTATGCTATACGAAG-3′) (49), and GFP (forward: 5′-TCTGAGTGGCAAAGGACCTTAGG-3′; reverse: 5′-CGCTGAACTTGTGGCCGTTTACG-3′) (37).
Hippocampal culture and transfection
Rat hippocampal cultures were prepared from embryonic day 18 (E18) embryos from Sprague-Dawley rats as previously described (12, 19). Cultures were maintained for 13 to 14 days in vitro (DIV) to assess presynaptic terminals or until 21 DIV to investigate dendritic spines or to analyze the impact of sFRP1 on presynaptic terminals.
Mouse hippocampal neurons were prepared from E15.5 to E16.6 WT or Lrp6-val mice and maintained until 12 to 21 DIV. Neurons were treated with recombinant Wnt7a (100 or 200 ng/ml; PeproTech, 120-31), recombinant Wnt3a (200 ng/ml; R&D Systems), or with bovine serum albumin (BSA; control vehicle) for 3 hours on DIV 12 for analyses of synapses.
Rat hippocampal neurons were transfected using either Amaxa nucleofection before plating or with calcium phosphate transfection at 7 to 9 DIV with DNA constructs expressing enhanced green fluorescent protein (EGFP)–actin, human LRP6 WT or human LRP6-Val, and MESD (mesoderm development LRP chaperone protein), which is required for maturation and trafficking of LRP6 (56). Control neurons were transfected with EGFP-actin only. Neurons (DIV 20) were treated with recombinant sFRP1 (1 μg/ml; R&D Systems) overnight for analyses of presynaptic terminals.
HeLa cell culture and transfection
HeLa cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco) and maintained at 37°C and 5% CO2. Cells were seeded on 12-mm glass coverslips at a density of 13.4 × 103 cells/cm2 for PLA assays, 13.95 × 103 cells/cm2 for analyses of pLRP6, and at 18.6 × 103 cells/cm2 for surface biotinylation.
Cells were transfected with plasmids encoding EGFP, Fz5-HA, MESD, and LRP6 WT or LRP6-Val using Lipofectamine 3000 (Invitrogen) and Opti-MEM for 4 hours according to the manufacturer’s protocol. The transfection medium was then replaced with Opti-MEM. Forty-eight hours after transfection, cells were treated with serum-free DMEM containing recombinant Wnt7a (200 ng/ml; PreproTech, 120-31) or BSA (control) for 30 or 60 min.
List of plasmids
LRP6 WT (Addgene, plasmid no. 27242) and Fz5-HA were gifts from X. He. MESD-Flag was a gift from B. Holdener. EGFP-actin was a gift from Y. Goda. LRP6-Val-HA was a gift from R. T. Moon. The untagged LRP6-Val plasmid used in this paper was generated using constructs provided by R. T. Moon.
Brain section preparation
For cryostat sections used for immunostaining, 4% paraformaldehyde (PFA) fixed brains were immersed in 30% sucrose and frozen in precooled 2-methylbutane. Thirty- to 50-μm sagittal sections were cut using a cryostat and stored at −20°C. Vibratome sections were prepared as previously described (57). Briefly, brains were rapidly dissected and immersed in artificial cerebrospinal fluid (ACSF), and 250- to 300-μm-thick sagittal brain slices were cut. Then, slices were fixed in 4% PFA/4% sucrose in phosphate-buffered saline (PBS).
Immunofluorescence staining
Immunofluorescence staining was performed as previously described (57). Slices were permeabilized and blocked in 0.5% Triton X-100 + 10% donkey serum in PBS for 3 to 4 hours at room temperature (RT) and then incubated with primary antibodies overnight at 4°C. Secondary antibodies (1:500; Alexa Fluor, Invitrogen) were incubated for 2 hours at RT. Slices were incubated in DAPI, washed with PBS, and mounted with Fluoromount-G (SouthernBiotech).
Cultured hippocampal neurons were fixed in 4% PFA with 4% sucrose in PBS for 20 min at RT. Neurons were permeabilized in 0.05% Triton X-100 in PBS for 5 min, blocked in 5% BSA for 1 hour, both at RT, and then incubated with primary antibodies overnight at 4°C. Secondary antibodies (1:600; Alexa Fluor, Invitrogen) were incubated for 1 hour at RT. Neurons were incubated with DAPI, washed with PBS, and mounted with FluorSave (Millipore).
Proximity ligation assay
PLA was performed according to the manufacturer’s protocol (Sigma-Aldrich). Briefly, cells were washed with PBS, fixed with warm 4% PFA for 15 min, and then blocked for 60 min at 37°C with the Duolink blocking solution. Cells were incubated overnight at 4°C with anti-LRP6 (R&D Systems, AF1505) and anti-HA (Sigma-Aldrich, H6908) primary antibodies in Duolink antibody diluent solution (1:800). Cells were washed three times with the Duolink wash buffer A and incubated with the anti-rabbit MINUS and anti-goat PLUS PLA probes in Duolink antibody diluent solution for 1 hour at 37°C. After two washes in wash buffer A, cells were incubated with the Duolink ligation solution for 30 min at 37°C. After another two wash buffer A washes, cells were incubated with the Duolink amplification solution for 1 hour 40 min at 37°C. Cells were washed 2 × 10 min in wash buffer B and for 1 min in 0.01× wash buffer B. Cells were then permeabilized for 10 min with 0.1% Triton X-100/PBS and blocked in 5% BSA for 1 hour. Anti-GFP (1:500; Millipore, 06-896) primary antibody was added for 1 hour at RT, followed by three PBS washes and the addition of Alexa Fluor 488 chicken for 1 hour at RT. After 3× PBS washes, coverslips were mounted using 5 μl per coverslip of Duolink in situ mounting medium with DAPI.
List of primary antibodies
The following primary antibodies were used: APP (6E10) (Novus Biotech, NBP2-62566, RRID:AB_2917960), Aβ (BioLegend, 803001, RRID:AB_2564653), β-actin (Cell Signaling Technology, 4970, RRID:AB_2223172), Bassoon (Novus Biologicals, NB120-13249), Bassoon (Synaptic Systems, 141016, RRID:AB_2661779), GFP (Millipore, 06-896, RRID:AB_310288), GFP (Invitrogen, A-6455), HA (Sigma-Aldrich, H6908, RRID:AB_260070), HA (Roche, 11867423001, RRID:AB_390918), Homer1, (Synaptic Systems, 160002, RRID:AB_2120990), Homer1, (Synaptic Systems, 160003, RRID:AB_887730), Homer1, (Synaptic Systems, 160006, RRID:AB_263122), LRP6 (Abcam, ab134146, RRID:AB_2895164), LRP6 (R&D Systems, AF1505, RRID:AB_2266025), LRP6 (Cell Signaling Technology, 2560, RRID:AB_2139329), LRP6 (Cell Signaling Technology, 3395, RRID:AB_1950408), pLRP6 (Cell Signaling Technology, 2568, RRID:AB_2139327), MAP2 (Abcam, ab5392, RRID:AB_2138153), MAP2 (Abcam, ab92434, RRID:AB_2138147), NeuN, (Cell Signaling Technology, 12943, RRID:AB_2630395), PSD-95 (Millipore, MAB1598, RRID:AB_94278), α-tubulin (Sigma-Aldrich, T9026, RRID:AB_477593), vGlut1 (Millipore, AB5905, RRID:AB_2301751), and vinculin (Sigma-Aldrich, V4505, RRID:AB_477617).
Confocal microscopy
Images were acquired on a Leica SP8 or an Olympus FV1000 inverted confocal microscope. For analyses of synaptic puncta and dendritic spines in brain sections, three images (stacks) were acquired per brain section, and three brain sections were analyzed per animal. For hippocampal cultures, 6 to 13 images (stacks) were acquired per condition. Each stack comprised 8 to 11 equidistant planes, 0.2 to 0.25 μm apart, and were acquired using a 63× 1.40–numerical aperture (NA) oil objective. For analyses of NeuN staining, a stack of 31 equidistant planes, 0.5 μm apart, was acquired for each brain section with a 10× 0.40-NA objective on a Leica SP8. For plaque analyses of 2-month-old mice, images were acquired on a Leica SP5. For each brain section, one stack of 25 equidistant planes, 0.99 μm apart, was acquired using a 10× 0.30-NA objective. For analyses of plaques in 7- and 10-month-old mice, a tile scan per brain section was acquired with a 2× 0.75-NA objective on a Leica SP8. Each stack comprised 59 equidistant planes for 7-month-old mice and 144 equidistant planes for 10-month-old mice, 0.35 μm apart. For the PLA experiments and analyses of pLRP6 in HeLa cells, stacks of 9 to 10 equidistant planes with a z step of 0.5 μm were acquired using a 40× oil objective on a Leica SP8. Two to three coverslips per experimental condition were imaged with two to five images per coverslip acquired.
Structured illumination microscopy
SIM was performed on a Zeiss Elyra S.1 microscope with a 63× oil-immersion objective (NA 1.40). Z-stacks of 15 to 20 equidistant planes were acquired. For each field of view, nine images were acquired using three different rotations and phases of structured illumination (a grid pattern) on the sample.
Electron microscopy
Sagittal brain sections of 200-μm thickness were cut on a vibratome and then fixed in 2% PFA/2.5% glutaraldehyde solution overnight at 4°C. Samples were postfixed in 1% OsO4 for 1 hour at 4°C and stained with 1% thiocarbohydrazide for 20 min at RT, 2% OsO4 for 30 min at RT, 1% uranyl acetate overnight at 4°C, and lead aspartate for 30 min at 60°C. Next, slices were dehydrated in graded alcohol and embedded in resin. Ultrathin sections (70 nm) were then cut using a diamond ultra 45° knife (Diatome) on a Leica UC7 ultramicrotome and collected on 2 mm–by–1 mm slot grids. Images were acquired on a transmission electron microscope (T12 Tecnai Spirit Bio-Twin, FEI), each covering 5.8 μm2 at 0.77 nm/pixel.
Image analyses
Image analyses were performed using Volocity software (PerkinElmer). For hippocampal cultures and brain sections, customized thresholding protocols were used to detect pre- and postsynaptic puncta. Synapses were quantified as colocalized pre- and postsynaptic puncta. Analyses of dendrite spines were performed blind to the genotype or treatment. Dendritic spines were measured manually along three to four sections of dendrite. Spine size was quantified using the line tool by measuring the maximum spine head width. For the PLA experiment, only transfected cells were selected (on the basis of GFP signal), and the total PLA signal intensity was quantified and divided by the number of cells analyzed. For analyses of pLRP6 levels, cells expressing GFP and LRP6 were analyzed. Total pLRP6 intensity was normalized to total LRP6 intensity in each cell. For SIM images, synapses were identified manually, and the colocalization of LRP6 with synaptic markers was determined using Volocity. For neuronal number, the number of NeuN and DAPI-positive cells was quantified using Volocity and divided by the total number of DAPI-positive cells. In NL-G-F and NL-G-F;Lrp6-val mice, plaques were manually counted at 2 months, blind to the genotype. At 7 months, plaque analyses were performed, blind to the genotype, using Fiji as previously described (58). Images were thresholded, and then, the particle analysis tool was used to count the number of plaques and the percent coverage area of Aβ. For EM, high-magnification images were used to manually count synaptic vesicles within 200 nm of the active zone. PSD length was quantified using a line tool in Fiji. Vesicle number was normalized to PSD length.
Electrophysiological recordings
Electrophysiological recordings were performed in 7- to 8-month-old and in 12- to 13-month-old male and female mice. Acute transverse hippocampal slices (300 μm thick) from WT control mice and homozygous Lrp6-val mice were cut with a Leica VT-1000 vibratome in ice-cold ACSF bubbled with 95% O2/5% CO2 containing NaCl (125 mM), KCl (2.4 mM), NaHCO3 (26 mM), NaH2PO4 (1.4 mM), d-(+)-glucose (20 mM), CaCl2 (0.5 mM), and MgCl2 (3 mM). Brain slices from 12- to 13-month-old mice were then transferred for 5 min into a series of three different oxygenated (95% O2/5% CO2) chambers in the same ACSF base but with gradual temperature and component variations: (i) 21°C, MgCl2 (1 mM) and CaCl2 (0.5 mM) and then placed at 36°C for 5 min; (ii) 36°C, MgCl2 (1 mM) and CaCl2 (1 mM); and (iii) 36°C with MgCl2 (1 mM) and CaCl2 (2 mM) before cooling to 21°C for at least 1 hour before recordings. Brain slices were placed in a chamber on an upright microscope and constantly perfused with NaCl (125 mM), KCl (2.4 mM), NaHCO3 (26 mM), NaH2PO4 (1.4 mM), d-(+)-glucose (20 mM), MgCl2 (1 mM), and CaCl2 (2 mM) supplemented with 10 μM bicuculline to block γ-aminobutyric acid currents at RT.
Whole-cell patch-clamp recordings were made from pyramidal cells in the CA1 region voltage-clamped at −60 mV using patch pipettes with a resistance of 4 to 8 megohm when filled with a cesium gluconate pipette solution composed of d-gluconic acid lactone (130 mM), Hepes (10 mM), EGTA (10 mM), NaCl (10 mM), CaCl2 (0.5 mM), MgCl2 (1 mM), adenosine 5′-triphosphate (1 mM), and guanosine 5′-triphosphate (0.5 mM), and QX314 (5 mM) adjusted to pH 7.2 with CsOH. To evoke postsynaptic currents, a bipolar concentric stimulating electrode (FHC) connected to a Grass S48 stimulator was placed around 100 to 200 μm from the patched cell. Cell I/O recordings were made with the stimulus pulse varied between 9 and 50 V with a pulse width of 0.1 ms and delivered at a rate of 0.1 Hz. At least three responses per stimulus intensity were averaged per cell. PPR stimuli were delivered at a rate of 0.2 Hz with varying ISIs, ranging from 50 to 200 ms. The stimulus intensity was adjusted for each cell to elicit ∼50% of the maximal response. PPR was calculated as the ratio of the peak amplitude of the second response over the first response, and at least seven responses were averaged per cell for each ISI. For RRP size, initial fusion efficiency, and SV recycling rate calculation, CA1 cell EPSCs were recorded in response to 3-s duration trains of stimulation at 20 Hz and estimated as previously described (40, 43).
The following two equations were used to estimate RRP size, fusion efficiency (fe), and vesicle recycling rate (α) using cumulative charge (43) in analysis of 20-Hz stimulus-evoked trains of EPSCs
| (1) |
| (2) |
where r(1) is the charge of the first EPSC in the train, r(i) is the charge passed by the ith EPSC, r(∞) was calculated from the average charge of the last 10 EPSCs in the train, and Δt is the stimulus interval in the train. The RRP was estimated as RRP = r (1)/fe.
Currents were recorded using an Axopatch 200B amplifier and low-pass–filtered at 1 kHz and digitized at 10 kHz. Online monitoring of the data was performed using WinEDR and offline analysis using both WinEDR and WinWCP software (freely available online at http://spider.science.strath.ac.uk/sipbs/software_ses.htm).
Surface biotinylation and Western blots
Surface biotinylation was performed using Sulfo-NHS-LC-LC-Biotin (Thermo Fisher Scientific, EZ-Link Sulfo-NHS-LC-LC-Biotin) and streptavidin agarose beads (Thermo Fisher Scientific). Samples were run on an 8% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gel. Hippocampal homogenates from WT and Lrp6-val mice at 4 months old were resolved on 8 to 12% SDS-PAGE gels. Two bands were observed for Fz5-HA. Both bands were quantified as these bands are likely to represent changes in the glycosylation of this receptor at the surface (59).
Enzyme-linked immunosorbent assay
NL-G-F and NL-G-F;Lrp6-val hippocampal tissue was homogenized in radioimmunoprecipitation assay (RIPA) buffer, followed by guanidine hydrochloride. Aβ42 peptides were quantified using a human Aβ 1-42 ELISA kit (Wako) following the manufacturer’s instructions.
Quantitative polymerase chain reaction
RNA was extracted from frozen hippocampal tissue using TRIzol (Life Technologies) and the Direct-zol RNA MiniPrep Kit (Zymo Research), following the manufacturer’s instructions. First-strand complementary DNA (cDNA) synthesis was performed with the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific), following the manufacturer’s instructions. qPCR was performed using GoTaq qPCR Master Mix (Promega). Gapdh, Canx, or Rpl13a was used as housekeeping genes. The following primers were used: Lrp6 (forward: 5′-TCTTGTGGTTGTCTGGTGTGGAG-3′; reverse: 5′-AGAAGACATATCAGAAAATGCAGGAGG-3′ or forward: 5′-AAGCTGCTGGAGAATGGAAA-3′; reverse: 5′-CCAAAGAAATTCGCCTCAAG-3′), Axin2 (forward: 5′-GAGGGACAGGAACCACTCG-3′; reverse: 5′-TGCCAGTTTCTTTGGCTCTT-3′), Fz5 (forward: 5′-ACATGGAACGATTCCGCTAC-3′; reverse: 5′-TCCCAGTGACACACACAGGT-3′), Wnt7a (forward: 5′-TTTCTCAGCCTGGGCATAGT-3′; reverse: 5′-CCAGAGCTACCACCGAAGAG-3′), Wnt7b (forward: 5′-GCCTTCACCTATGCCATCAC-3′; reverse: 5′-CCTTCCGCCTGGTTGTAGTA-3′), Gapdh (forward: 5′-CGTCCCGTAGACAAAATGGT-3′; reverse: 5′-TCAATGAAGGGGTCGTTGAT-3′ or forward: 5′-AGACAGCCGCATCTTCTTGT-3′; reverse: 5′-CTTGCCGTGGGTAGAGTCAT-3′), Canx (forward: 5′-CCCACATAGGAGGTCTGACA-3′; reverse: 5′-GCTAGGAATGGAGGAGATCCA-3′), and Rpl13a (forward: 5′-GACTCCTGGTGTGAACCCA-3′; reverse: 5′-CTCTACCCACAGGAGCAGT-3′).
Statistical analyses
All results are presented as means ± SEM. Statistical analyses were performed in GraphPad Prism (version 9) or SPSS (IBM, version 27). Normality was assessed with Shapiro-Wilk or Kolmogorov-Smirnov tests. Normally distributed data were analyzed using t tests for two conditions, one-way analysis of variance (ANOVA) for two or more conditions, or two-way ANOVA for experiments with two independent variables. Post hoc tests are detailed in the figure legends. Nonnormally distributed data were analyzed with nonparametric tests such as Kruskal-Wallis or Mann-Whitney. Statistical significance was accepted as *P < 0.05, **P < 0.01, and ***P < 0.001.
Acknowledgments
We would like to thank T. Saito and T. Saido for the NL-G-F mice; P. Caroni for the Thy1-GFP mice; X. He, R. Moon, B. Holdener, and Y. Goda for DNA constructs; and F. Brodsky for the cell lines. We would like to thank I. White for performing the EM. We would like to thank members of our laboratory for discussions on the data and comments on our manuscript. Diagrams were created with BioRender.com.
Funding: This work was supported by MRC MR/M024083/1 and MR/S012125/1 (to P.C.S.), ARUK ARUK-PPG2021B/030 (to P.C.S.), the Alzheimer’s Society AS-PG-17-006 (to P.C.S.), MRC MC_ST_LMCB_2019 (to M.E.J.), and the Wellcome Trust 102267/Z/13/Z (to J.B.).
Author contributions: Conceptualization: P.C.S. Methodology: E.M., K.B., M.E.J., J.B., T.D., N.M.-F., S.T., and E.P. Investigation: M.E.J., J.B., T.D., N.M.-F., S.T., and E.P. Visualization: M.E.J., J.B., T.D., N.M.-F., S.T., and E.P. Supervision: P.C.S. and A.G. Writing—original draft: P.C.S., M.E.J., and J.B. Writing—review and editing: P.C.S., M.E.J., J.B., A.G., T.D., E.M., N.M.-F., S.T., and E.P.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The Thy1-GFP and NL-G-F mice can be provided by P. Caroni and T. Saido, respectively. Please note that a completed material transfer agreement might be required. Requests for the mouse lines should be submitted to P. Caroni and T. Saido, respectively.
Supplementary Materials
This PDF file includes:
Figs. S1 to S12
REFERENCES AND NOTES
- 1.R. D. Terry, E. Masliah, D. P. Salmon, N. Butters, R. DeTeresa, R. Hill, L. A. Hansen, R. Katzman, Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991). [DOI] [PubMed] [Google Scholar]
- 2.S. W. Scheff, D. A. Price, Alzheimer’s disease-related alterations in synaptic density: Neocortex and hippocampus. J. Alzheimers Dis. 9, 101–115 (2006). [DOI] [PubMed] [Google Scholar]
- 3.G. M. Shankar, D. M. Walsh, Alzheimer’s disease: Synaptic dysfunction and Aβ. Mol. Neurodegener. 4, 48 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.L. Mucke, D. J. Selkoe, Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2, a006338 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.R. M. Koffie, T. Hashimoto, H.-C. Tai, K. R. Kay, A. Serrano-Pozo, D. Joyner, S. Hou, K. J. Kopeikina, M. P. Frosch, V. M. Lee, D. M. Holtzman, B. T. Hyman, T. L. Spires-Jones, Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-β. Brain 135, 2155–2168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.R. M. Koffie, M. Meyer-Luehmann, T. Hashimoto, K. W. Adams, M. L. Mielke, M. Garcia-Alloza, K. D. Micheva, S. J. Smith, M. L. Kim, V. M. Lee, B. T. Hyman, T. L. Spires-Jones, Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. U.S.A. 106, 4012–4017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.R. J. Jackson, N. Rudinskiy, A. G. Herrmann, S. Croft, J. S. M. Kim, V. Petrova, J. J. Ramos-Rodriguez, R. Pitstick, S. Wegmann, M. Garcia-Alloza, G. A. Carlson, B. T. Hyman, T. L. Spires-Jones, Human tau increases amyloid β plaque size but not amyloid β-mediated synapse loss in a novel mouse model of Alzheimer’s disease. Eur. J. Neurosci. 44, 3056–3066 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.H. Dong, M. V. Martin, S. Chambers, J. G. Csernansky, Spatial relationship between synapse loss and β-amyloid deposition in Tg2576 mice. J. Comp. Neurol. 500, 311–321 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D. J. Selkoe, J. Hardy, The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Y.-C. Lin, A. J. Koleske, Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu. Rev. Neurosci. 33, 349–378 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Y. Chen, A. K. Y. Fu, N. Y. Ip, Synaptic dysfunction in Alzheimer’s disease: Mechanisms and therapeutic strategies. Pharmacol. Ther. 195, 186–198 (2019). [DOI] [PubMed] [Google Scholar]
- 12.A. Marzo, S. Galli, D. Lopes, F. McLeod, M. Podpolny, M. Segovia-Roldan, L. Ciani, S. Purro, F. Cacucci, A. Gibb, P. C. Salinas, Reversal of synapse degeneration by restoring Wnt signaling in the adult hippocampus. Curr. Biol. 26, 2551–2561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.C.-C. Liu, C.-W. Tsai, F. Deak, J. Rogers, M. Penuliar, Y. M. Sung, J. N. Maher, Y. Fu, X. Li, H. Xu, S. Estus, H.-S. Hoe, J. D. Fryer, T. Kanekiyo, G. Bu, Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer’s disease. Neuron 84, 63–77 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.S. A. Purro, E. M. Dickins, P. C. Salinas, The secreted Wnt antagonist Dickkopf-1 is required for amyloid β-mediated synaptic loss. J. Neurosci. 32, 3492–3498 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.M. Sahores, A. Gibb, P. C. Salinas, Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development 137, 2215–2225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.E. K. Davis, Y. Zou, A. Ghosh, Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Dev. 3, 32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.W. Cerpa, J. A. Godoy, I. Alfaro, G. G. Farías, M. J. Metcalfe, R. Fuentealba, C. Bonansco, N. C. Inestrosa, Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J. Biol. Chem. 283, 5918–5927 (2008). [DOI] [PubMed] [Google Scholar]
- 18.L. Ciani, K. A. Boyle, E. Dickins, M. Sahores, D. Anane, D. M. Lopes, A. J. Gibb, P. C. Salinas, Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca2+/calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. U.S.A. 108, 10732–10737 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.F. McLeod, A. Bossio, A. Marzo, L. Ciani, S. Sibilla, S. Hannan, G. A. Wilson, E. Palomer, T. G. Smart, A. Gibb, P. C. Salinas, Wnt Signaling Mediates LTP-Dependent Spine Plasticity and AMPAR localization through Frizzled-7 receptors. Cell Rep. 23, 1060–1071 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.K. Sharma, S.-Y. Choi, Y. Zhang, T. J. F. Nieland, S. Long, M. Li, R. L. Huganir, High-throughput genetic screen for synaptogenic factors: Identification of LRP6 as critical for excitatory synapse development. Cell Rep. 5, 1330–1341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.S. Fazel Darbandi, S. E. Robinson Schwartz, E. L.-L. Pai, A. Everitt, M. L. Turner, B. N. R. Cheyette, A. J. Willsey, M. W. State, V. S. Sohal, J. L. R. Rubenstein, Enhancing WNT signaling restores cortical neuronal spine maturation and synaptogenesis in Tbr1 mutants. Cell Rep. 31, 107495 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.S. Galli, D. M. Lopes, R. Ammari, J. Kopra, S. E. Millar, A. Gibb, P. C. Salinas, Deficient Wnt signalling triggers striatal synaptic degeneration and impaired motor behaviour in adult mice. Nat. Commun. 5, 4992 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.C.-M. Chen, L. L. Orefice, S.-L. Chiu, T. LeGates, S. Hattar, R. L. Huganir, H. Zhao, B. Xu, R. Kuruvilla, Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. Proc. Natl. Acad. Sci. U.S.A. 114, E619–E628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A. Caricasole, A. Copani, F. Caraci, E. Aronica, A. J. Rozemuller, A. Caruso, M. Storto, G. Gaviraghi, G. C. Terstappen, F. Nicoletti, Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J. Neurosci. 24, 6021–6027 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.M. C. Rosi, I. Luccarini, C. Grossi, A. Fiorentini, M. G. Spillantini, A. Prisco, C. Scali, M. Gianfriddo, A. Caricasole, G. C. Terstappen, F. Casamenti, Increased Dickkopf-1 expression in transgenic mouse models of neurodegenerative disease. J. Neurochem. 112, 1539–1551 (2010). [DOI] [PubMed] [Google Scholar]
- 26.K. J. Sellers, C. Elliott, J. Jackson, A. Ghosh, E. Ribe, A. I. Rojo, H. H. Jarosz-Griffiths, I. A. Watson, W. Xia, M. Semenov, P. Morin, N. M. Hooper, R. Porter, J. Preston, R. al-Shawi, G. Baillie, S. Lovestone, A. Cuadrado, M. Harte, P. Simons, D. P. Srivastava, R. Killick, Amyloid β synaptotoxicity is Wnt-PCP dependent and blocked by fasudil. Alzheimers Dement. 14, 306–317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R. Killick, E. M. Ribe, R. al-Shawi, B. Malik, C. Hooper, C. Fernandes, R. Dobson, P. M. Nolan, A. Lourdusamy, S. Furney, K. Lin, G. Breen, R. Wroe, A. W. M. To, K. Leroy, M. Causevic, A. Usardi, M. Robinson, W. Noble, R. Williamson, K. Lunnon, S. Kellie, C. H. Reynolds, C. Bazenet, A. Hodges, J.-P. Brion, J. Stephenson, J. Paul Simons, S. Lovestone, Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol. Psychiatry 19, 88–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.G. V. De Ferrari, A. Papassotiropoulos, T. Biechele, F. W. De-Vrieze, M. E. Avila, M. B. Major, A. Myers, K. Sáez, J. P. Henríquez, A. Zhao, M. A. Wollmer, R. M. Nitsch, C. Hock, C. M. Morris, J. Hardy, R. T. Moon, Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 104, 9434–9439 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.M. A. Alarcón, M. A. Medina, Q. Hu, M. E. Avila, B. I. Bustos, E. Pérez-Palma, A. Peralta, P. Salazar, G. D. Ugarte, A. E. Reyes, G. M. Martin, C. Opazo, R. T. Moon, G. V. De Ferrari, A novel functional low-density lipoprotein receptor-related protein 6 gene alternative splice variant is associated with Alzheimer’s disease. Neurobiol. Aging 34, 1709.e9–1809.e9 (2013). [DOI] [PubMed] [Google Scholar]
- 30.1000 Genomes Project Consortium, A. Auton, L. D. Brooks, R. M. Durbin, E. P. Garrison, H. M. Kang, J. O. Korbel, J. L. Marchini, S. M. Carthy, G. A. M. Vean, G. R. Abecasis, A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.E. Bourhis, C. Tam, Y. Franke, J. F. Bazan, J. Ernst, J. Hwang, M. Costa, A. G. Cochran, R. N. Hannoush, Reconstitution of a Frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J. Biol. Chem. 285, 9172–9179 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.S. Chen, D. Bubeck, B. T. MacDonald, W. X. Liang, J.-H. Mao, T. Malinauskas, O. Llorca, A. R. Aricescu, C. Siebold, X. He, E. Y. Jones, Structural and functional studies of LRP6 ectodomain reveal a platform for Wnt signaling. Dev. Cell 21, 848–861 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.H. Hirai, K. Matoba, E. Mihara, T. Arimori, J. Takagi, Crystal structure of a mammalian Wnt-frizzled complex. Nat. Struct. Mol. Biol. 26, 372–379 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Y. Hua, Y. Yang, Q. Li, X. He, W. Zhu, J. Wang, X. Gan, Oligomerization of Frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/β-catenin pathway. J. Biol. Chem. 293, 19710–19724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.B. Mao, W. Wu, Y. Li, D. Hoppe, P. Stannek, A. Glinka, C. Niehrs, LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325 (2001). [DOI] [PubMed] [Google Scholar]
- 36.E. Ramos-Fernández, C. Tapia-Rojas, V. T. Ramírez, N. C. Inestrosa, Wnt-7a stimulates dendritic spine morphogenesis and PSD-95 expression through canonical signaling. Mol. Neurobiol. 56, 1870–1882 (2019). [DOI] [PubMed] [Google Scholar]
- 37.G. Feng, R. H. Mellor, M. Bernstein, C. Keller-Peck, Q. T. Nguyen, M. Wallace, J. M. Nerbonne, J. W. Lichtman, J. R. Sanes, Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000). [DOI] [PubMed] [Google Scholar]
- 38.F. McLeod, P. C. Salinas, Wnt proteins as modulators of synaptic plasticity. Curr. Opin. Neurobiol. 53, 90–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.C. A. Oliva, C. Montecinos-Oliva, N. C. Inestrosa, Wnt signaling in the central nervous system: New insights in health and disease. Prog. Mol. Biol. Transl. Sci. 153, 81–130 (2018). [DOI] [PubMed] [Google Scholar]
- 40.L. Ciani, A. Marzo, K. Boyle, E. Stamatakou, D. M. Lopes, D. Anane, F. McLeod, S. B. Rosso, A. Gibb, P. C. Salinas, Wnt signalling tunes neurotransmitter release by directly targeting Synaptotagmin-1. Nat. Commun. 6, 8302 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.L. E. Dobrunz, C. F. Stevens, Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18, 995–1008 (1997). [DOI] [PubMed] [Google Scholar]
- 42.D. Fioravante, W. G. Regehr, Short-term forms of presynaptic plasticity. Curr. Opin. Neurobiol. 21, 269–274 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.J. F. Wesseling, D. C. Lo, Limit on the role of activity in controlling the release-ready supply of synaptic vesicles. J. Neurosci. 22, 9708–9720 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.E. Jho, T. Zhang, C. Domon, C.-K. Joo, J.-N. Freund, F. Costantini, Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172–1183 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.L. Varela-Nallar, C. P. Grabowski, I. E. Alfaro, A. R. Alvarez, N. C. Inestrosa, Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function. Neural Dev. 4, 41 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O. Söderberg, M. Gullberg, M. Jarvius, K. Ridderstråle, K.-J. Leuchowius, J. Jarvius, K. Wester, P. Hydbring, F. Bahram, L. G. Larsson, U. Landegren, Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 (2006). [DOI] [PubMed] [Google Scholar]
- 47.A. Klaesson, K. Grannas, T. Ebai, J. Heldin, B. Koos, M. Leino, D. Raykova, J. Oelrich, L. Arngården, O. Söderberg, U. Landegren, Improved efficiency of in situ protein analysis by proximity ligation using UnFold probes. Sci. Rep. 8, 5400 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.C. Niehrs, J. Shen, Regulation of Lrp6 phosphorylation. Cell. Mol. Life Sci. 67, 2551–2562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.T. Saito, Y. Matsuba, N. Mihira, J. Takano, P. Nilsson, S. Itohara, N. Iwata, T. C. Saido, Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 17, 661–663 (2014). [DOI] [PubMed] [Google Scholar]
- 50.J. B. J. van Meurs, F. Rivadeneira, M. Jhamai, W. Hugens, A. Hofman, J. P. T. M. van Leeuwen, H. A. P. Pols, A. G. Uitterlinden, Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J. Bone Miner. Res. 21, 141–150 (2006). [DOI] [PubMed] [Google Scholar]
- 51.R. Sarzani, F. Salvi, M. Bordicchia, F. Guerra, I. Battistoni, G. Pagliariccio, L. Carbonari, P. Dessì-Fulgheri, A. Rappelli, Carotid artery atherosclerosis in hypertensive patients with a functional LDL receptor-related protein 6 gene variant. Nutr. Metab. Cardiovasc. Dis. 21, 150–156 (2011). [DOI] [PubMed] [Google Scholar]
- 52.K. I. Pinson, J. Brennan, S. Monkley, B. J. Avery, W. C. Skarnes, An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538 (2000). [DOI] [PubMed] [Google Scholar]
- 53.A. M. M. Orellana, A. R. Vasconcelos, J. A. Leite, L. de Sá Lima, D. Z. Andreotti, C. D. Munhoz, E. M. Kawamoto, C. Scavone, Age-related neuroinflammation and changes in Akt-GSK-3β and WNT/ β-catenin signaling in rat hippocampus. Aging (Albany, NY). 7, 1094–1111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.J. Folke, B. Pakkenberg, T. Brudek, Impaired wnt signaling in the prefrontal cortex of alzheimer’s disease. Mol. Neurobiol. 56, 873–891 (2019). [DOI] [PubMed] [Google Scholar]
- 55.L. Cong, F. A. Ran, D. Cox, S. Lin, R. Barretto, N. Habib, P. D. Hsu, X. Wu, W. Jiang, L. A. Marraffini, F. Zhang, Multiplex genome engineering using CRISPR-Cas systems. Science 339, 819–823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.J.-C. Hsieh, L. Lee, L. Zhang, S. Wefer, K. Brown, C. DeRossi, M. E. Wines, T. Rosenquist, B. C. Holdener, Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112, 355–367 (2003). [DOI] [PubMed] [Google Scholar]
- 57.F. McLeod, A. Marzo, M. Podpolny, S. Galli, P. Salinas, Evaluation of synapse density in hippocampal rodent brain slices. J. Vis. Exp. 6, 56153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.E. K. Pickett, A. G. Herrmann, J. M. Queen, K. Abt, O. Dando, J. Tulloch, P. Jain, S. Dunnett, S. Sohrabi, M. P. Fjeldstad, W. Calkin, L. Murison, R. J. Jackson, M. Tzioras, A. Stevenson, M. d’Orange, M. Hooley, C. Davies, M. Colom-Cadena, A. Anton-Fernandez, D. King, I. Oren, J. Rose, C.-A. M. Kenzie, E. Allison, C. Smith, O. Hardt, C. M. Henstridge, G. E. Hardingham, T. L. Spires-Jones, Amyloid beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimer’s disease. Cell Rep. 29, 3592–3604.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.B.-K. Koo, M. Spit, I. Jordens, T. Y. Low, D. E. Stange, M. van de Wetering, J. H. van Es, S. Mohammed, A. J. R. Heck, M. M. Maurice, H. Clevers, Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S12