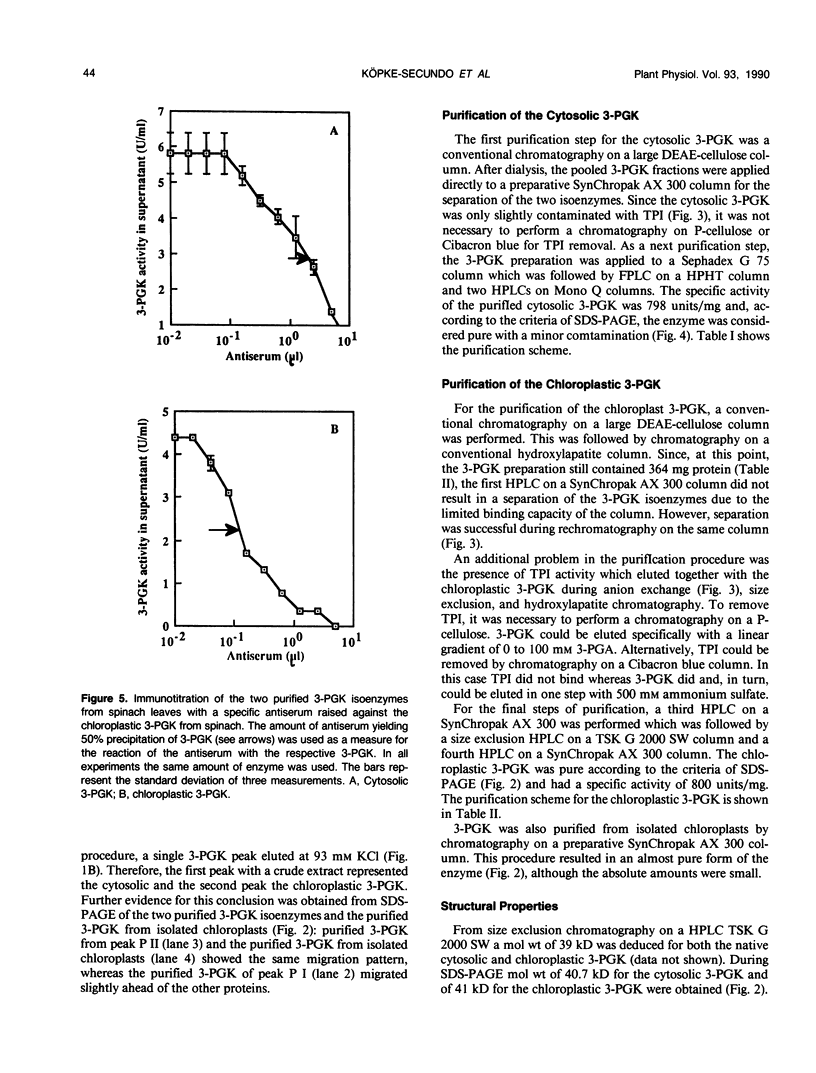
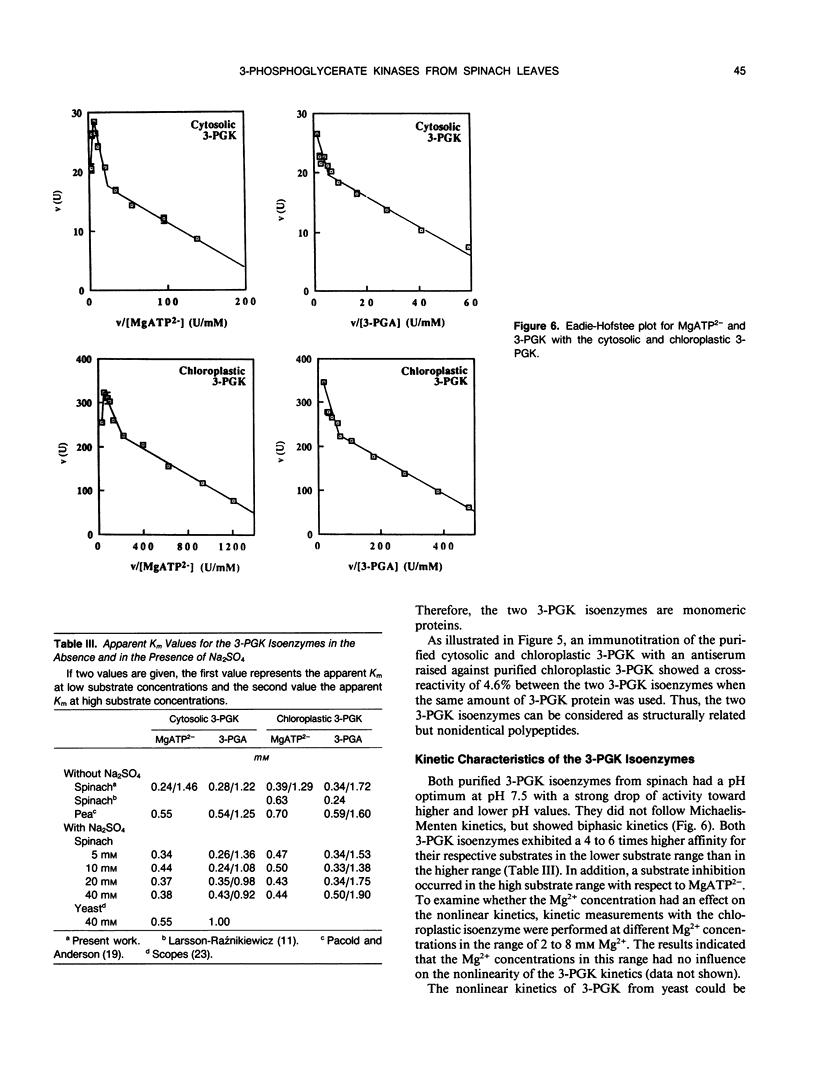
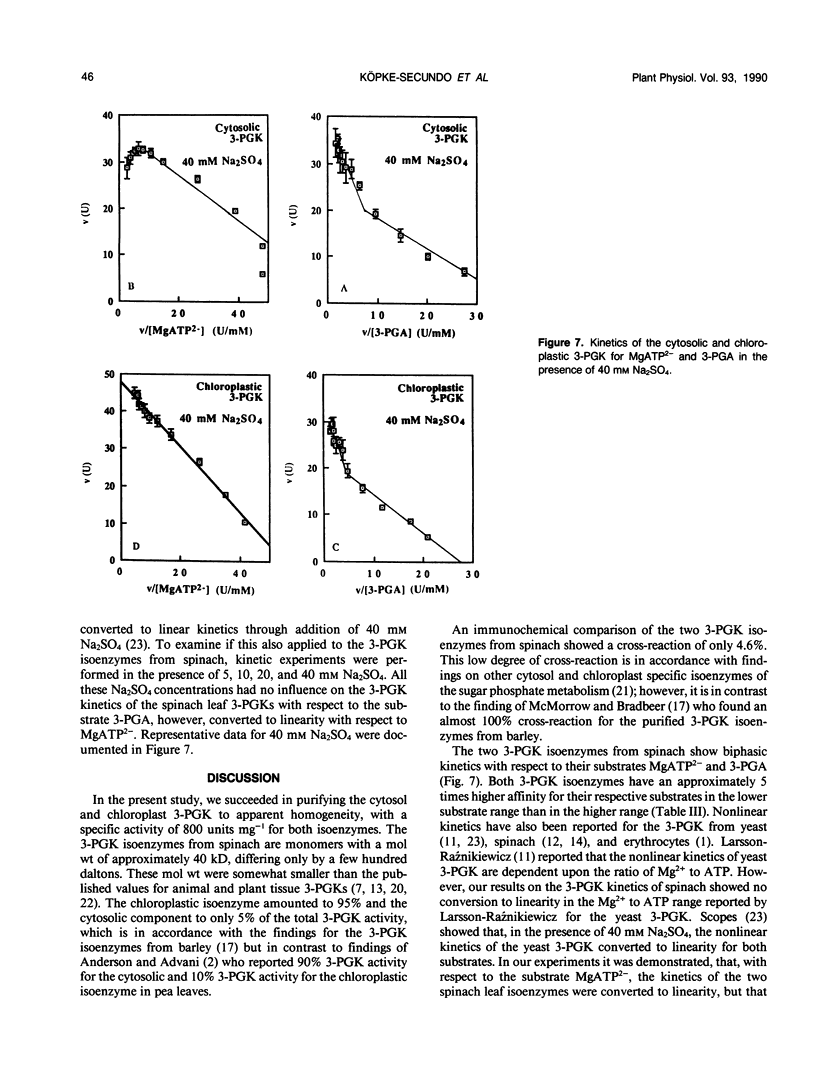
Abstract
The cytosol and chloroplast 3-phosphoglycerate kinases (3-PGK) from spinach (Spinacia oleracea L.) were purifled to apparent homogeneity. The procedure included a conventional anion-exchange chromatography on DEAE-cellulose and mainly a series of HPLC columns. The charge differences of the two isoenzymes were so small that separation was only successful by anion-exchange chromatography on a HPLC SynChropak AX 300 column. The portion of the two isoenzmyes in leaf tissue was estimated as 5% and 95%. The major 3-PGK was associated with isolated chloroplasts while the other 3-PGK was only found in the soluble cell fraction. The specific activity of the purified enzymes were in the order of 800 units (per milligram of protein). The molecular weight for the two 3-PGKs under nondenaturing (size exclusion chromatography) and denaturing (SDS-PAGE) conditions were in the order of 40 kilodaltons, with the cytosolic 3-PGK being slightly smaller than the chloroplastic 3-PGK. An antiserum against the chloroplastic 3-PGK showed only 4.6% cross-reaction of the chloroplastic 3-PGK with the cytosolic 3-PGK. The kinetics for glycerate-3-phosphate and MgATP2− were biphasic. The presence of Na2SO4 changed the MgATP2− dependence to linearity but not the glycerate-3-phosphate dependence.
Full text
PDF
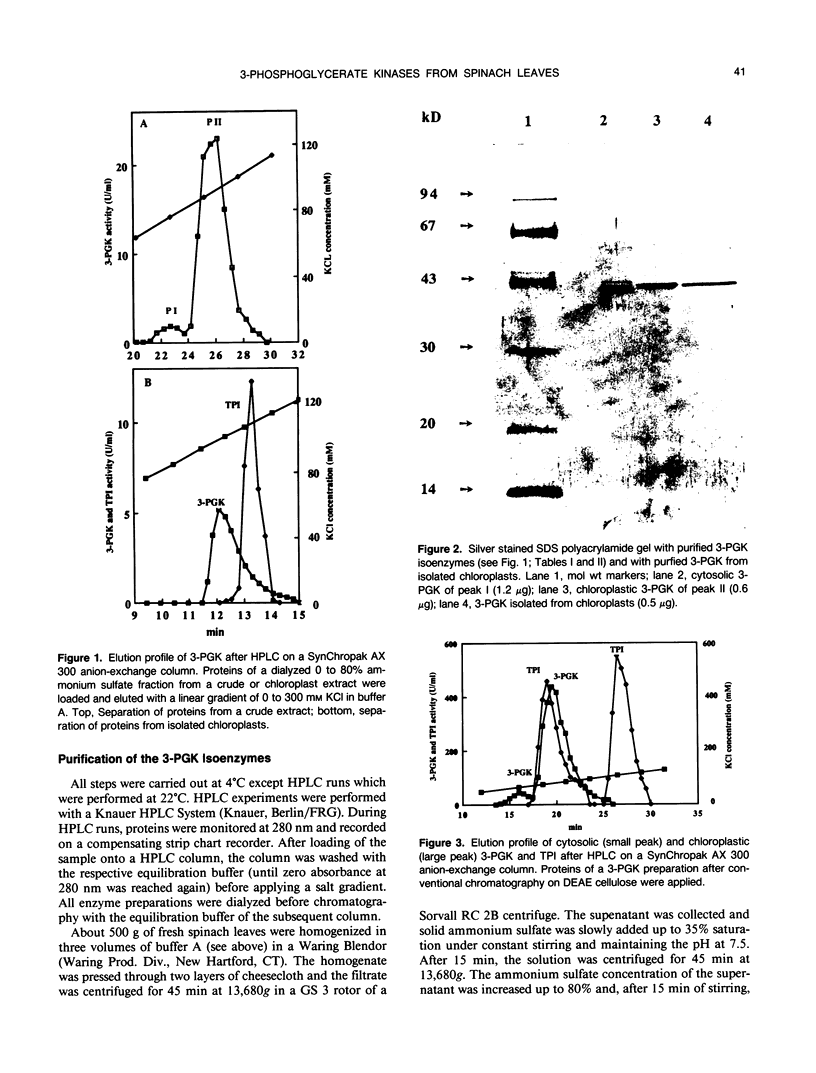


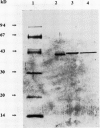
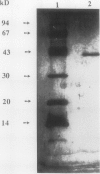
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., Brownstone Y. S. A study of phosphoglycerate kinase in human erythrocytes. II. Kinetic properties. Biochim Biophys Acta. 1976 Aug 12;445(1):89–103. doi: 10.1016/0005-2744(76)90162-5. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks R. D., Blake C. C., Evans P. R., Haser R., Rice D. W., Hardy G. W., Merrett M., Phillips A. W. Sequence, structure and activity of phosphoglycerate kinase: a possible hinge-bending enzyme. Nature. 1979 Jun 28;279(5716):773–777. doi: 10.1038/279773a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cavell S., Scopes K. Isolation and characterization of the 'photosynthetic' phosphoglycerate kinase from Beta vulgaris. Eur J Biochem. 1976 Apr 1;63(2):483–490. doi: 10.1111/j.1432-1033.1976.tb10251.x. [DOI] [PubMed] [Google Scholar]
- Fifis T., Scopes R. K. Purification of 3-phosphoglycerate kinase from diverse sources by affinity elution chromatography. Biochem J. 1978 Oct 1;175(1):311–319. doi: 10.1042/bj1750311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger I., Schnarrenberger C. Purification, subunit structure and immunological comparison of fructose-bisphosphate aldolases from spinach and corn leaves. Eur J Biochem. 1983 Oct 17;136(1):101–106. doi: 10.1111/j.1432-1033.1983.tb07711.x. [DOI] [PubMed] [Google Scholar]
- Kuntz G. W., Eber S., Kessler W., Krietsch H., Krietsch W. K. Isolation of phosphoglycerate kinases by affinity chromatography. Eur J Biochem. 1978 Apr 17;85(2):493–501. doi: 10.1111/j.1432-1033.1978.tb12265.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson-Raźnikiewicz M. Kinetic studies on the reaction catalyzed by phosphoglycerate kinase. II. The kinetic relationships between 3-phosphoglycerate, MgATP2-and activating metal ion. Biochim Biophys Acta. 1967 Jan 11;132(1):33–40. doi: 10.1016/0005-2744(67)90189-1. [DOI] [PubMed] [Google Scholar]
- Malhotra O. P., Kumar A., Tikoo K. Isolation and quaternary structure of a complex of glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase. Indian J Biochem Biophys. 1987 Oct;24(5):suppl–20. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Pacold I., Anderson L. E. Chloroplast and Cytoplasmic Enzymes: VI. Pea Leaf 3-Phosphoglycerate Kinases. Plant Physiol. 1975 Feb;55(2):168–171. doi: 10.1104/pp.55.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C. Regulation and structure of isozymes of sugar phosphate metabolism in plants. Isozymes Curr Top Biol Med Res. 1987;16:223–240. [PubMed] [Google Scholar]
- Scopes R. K. The steady-state kinetics of yeast phosphoglycerate kinase. Anomalous kinetic plots and the effects of salts on activity. Eur J Biochem. 1978 Apr 17;85(2):503–516. doi: 10.1111/j.1432-1033.1978.tb12266.x. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Metabolite transfer via enzyme-enzyme complexes. Science. 1986 Nov 28;234(4780):1081–1086. doi: 10.1126/science.3775377. [DOI] [PubMed] [Google Scholar]
- Tanswell P., Westhead E. W., Williams R. J. Nuclear-magnetic-resonance study of the active-site structure of yeast phosphoglycerate kinase. Eur J Biochem. 1976 Mar 16;63(1):249–262. doi: 10.1111/j.1432-1033.1976.tb10227.x. [DOI] [PubMed] [Google Scholar]
- Weber J. P., Bernhard S. A. Transfer of 1,3-diphosphoglycerate between glyceraldehyde-3-phosphate dehydrogenase and 3-phosphoglycerate kinase via an enzyme-substrate-enzyme complex. Biochemistry. 1982 Aug 17;21(17):4189–4194. doi: 10.1021/bi00260a042. [DOI] [PubMed] [Google Scholar]