Abstract
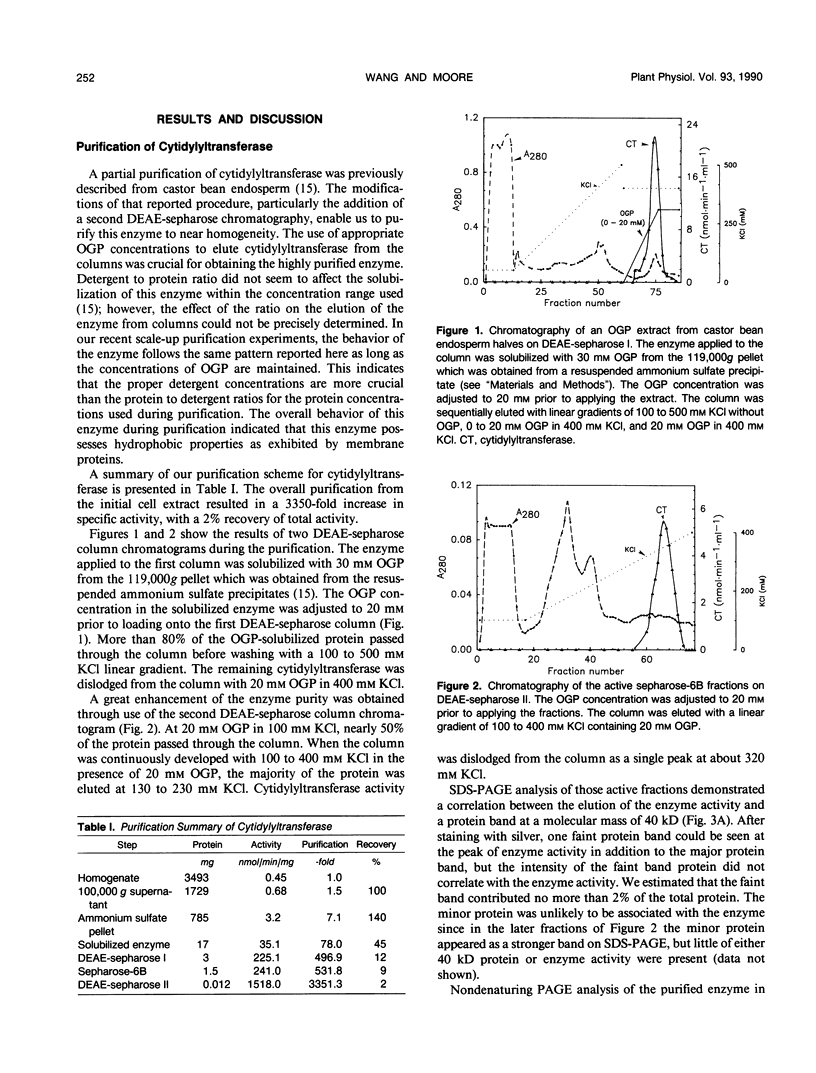
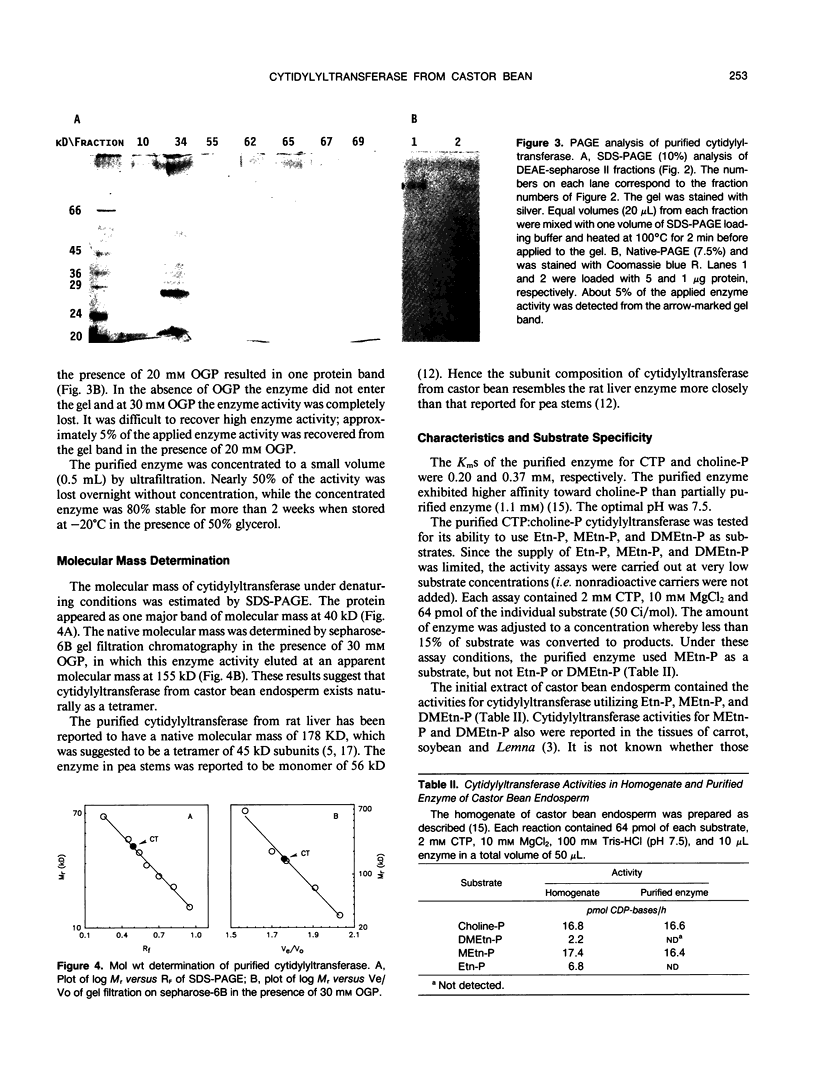
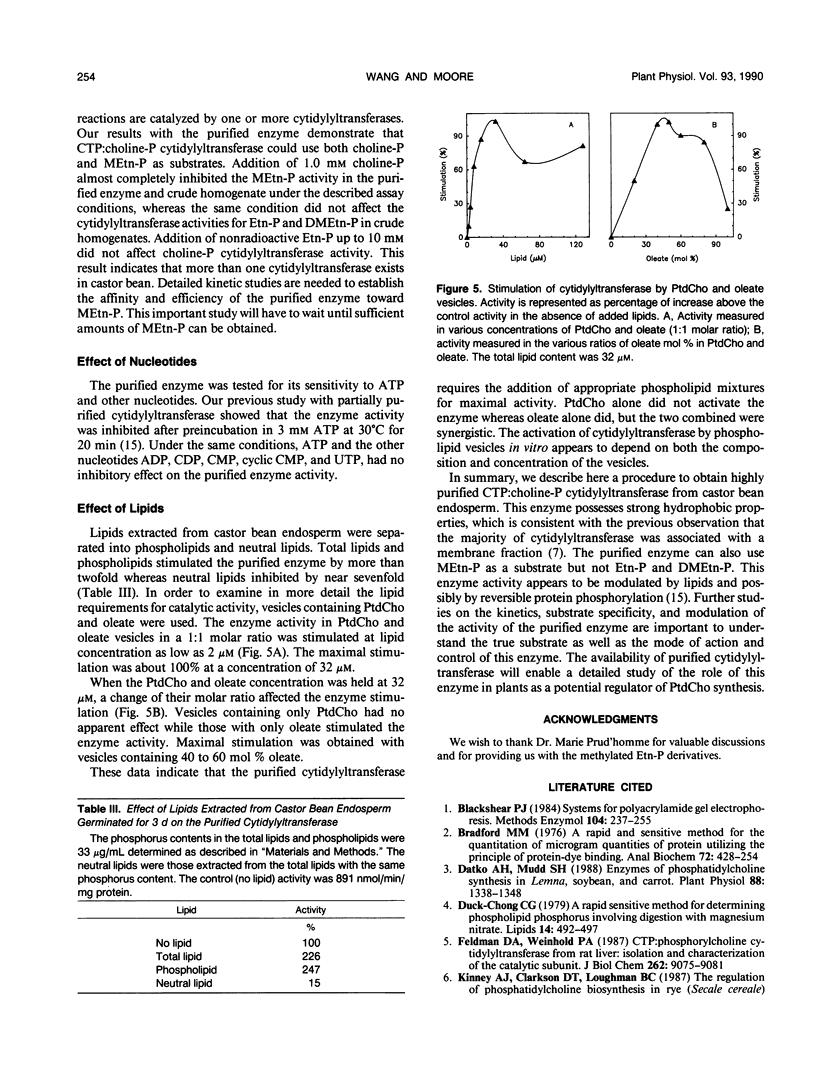
Cytidine 5′-triphosphate:choline-phosphate cytidylyltransferase (EC 2.7.7.15) has been purified to near homogeneity (3350-fold) from castor bean (Ricinus communis L. var Hale) endosperm. The steps of purification included a differential solubilization of this enzyme with n-octyl β-d-glucopyranoside (OGP) and column chromatography on sequential DEAE-sepharose, sepharose-6B, and second DEAE-sepharose columns. The uses of appropriate concentrations of the detergent, OGP, in each step were crucial to obtain the highly purified enzyme. The purified enzyme gave a single protein band on nondenaturing polyacrylamide gel electrophoresis. Sodium dodecyl sulfate polyacrylamide gel electrophoresis showed one major protein band of 40 kilodaltons. Gel filtration chromatography indicated that native cytidylyltransferase was approximately 155 kilodaltons, suggesting that it exists naturally as a tetramer. The purified enzyme used methylethanolamine-phosphate as a substrate but not ethanolamine-phosphate and dimethylethanolamine-phosphate. ATP and other nucleotides tested showed little effect on the purified enzyme. The purified enzyme activity was stimulated by both phospholipids extracted from castor bean endosperm and phosphatidylcholineoleate vesicles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackshear P. J. Systems for polyacrylamide gel electrophoresis. Methods Enzymol. 1984;104:237–255. doi: 10.1016/s0076-6879(84)04093-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Enzymes of phosphatidylcholine synthesis in lemna, soybean, and carrot. Plant Physiol. 1988 Dec;88(4):1338–1348. doi: 10.1104/pp.88.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D. A., Weinhold P. A. CTP:phosphorylcholine cytidylyltransferase from rat liver. Isolation and characterization of the catalytic subunit. J Biol Chem. 1987 Jul 5;262(19):9075–9081. [PubMed] [Google Scholar]
- Kinney A. J., Clarkson D. T., Loughman B. C. The regulation of phosphatidylcholine biosynthesis in rye (Secale cereale) roots. Stimulation of the nucleotide pathway by low temperature. Biochem J. 1987 Mar 15;242(3):755–759. doi: 10.1042/bj2420755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney A. J., Moore T. S., Jr Phosphatidylcholine synthesis in castor bean endosperm: the localization and control of CTP: choline-phosphate cytidylyltransferase activity. Arch Biochem Biophys. 1987 Nov 15;259(1):15–21. doi: 10.1016/0003-9861(87)90464-4. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Datko A. H. Phosphoethanolamine bases as intermediates in phosphatidylcholine synthesis by lemna. Plant Physiol. 1986 Sep;82(1):126–135. doi: 10.1104/pp.82.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Jun 25;779(2):217–251. doi: 10.1016/0304-4157(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Price-Jones M. J., Harwood J. L. Hormonal regulation of phosphatidylcholine synthesis in plants. The inhibition of cytidylyltransferase activity by indol-3-ylacetic acid. Biochem J. 1983 Dec 15;216(3):627–631. doi: 10.1042/bj2160627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Jones M. J., Harwood J. L. The control of CTP:choline-phosphate cytidylyltransferase activity in pea (Pisum sativum L.). Biochem J. 1986 Dec 15;240(3):837–842. doi: 10.1042/bj2400837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera J. S., Vance D. E. CTP:phosphocholine cytidylyltransferase is a substrate for cAMP-dependent protein kinase in vitro. J Biol Chem. 1989 Jan 15;264(2):1215–1223. [PubMed] [Google Scholar]
- Wang X. M., Moore T. S., Jr Partial purification and characterization of CTP:cholinephosphate cytidylyltransferase from castor bean endosperm. Arch Biochem Biophys. 1989 Nov 1;274(2):338–347. doi: 10.1016/0003-9861(89)90447-5. [DOI] [PubMed] [Google Scholar]
- Weinhold P. A., Rounsifer M. E., Feldman D. A. The purification and characterization of CTP:phosphorylcholine cytidylyltransferase from rat liver. J Biol Chem. 1986 Apr 15;261(11):5104–5110. [PubMed] [Google Scholar]



