Figure 8.
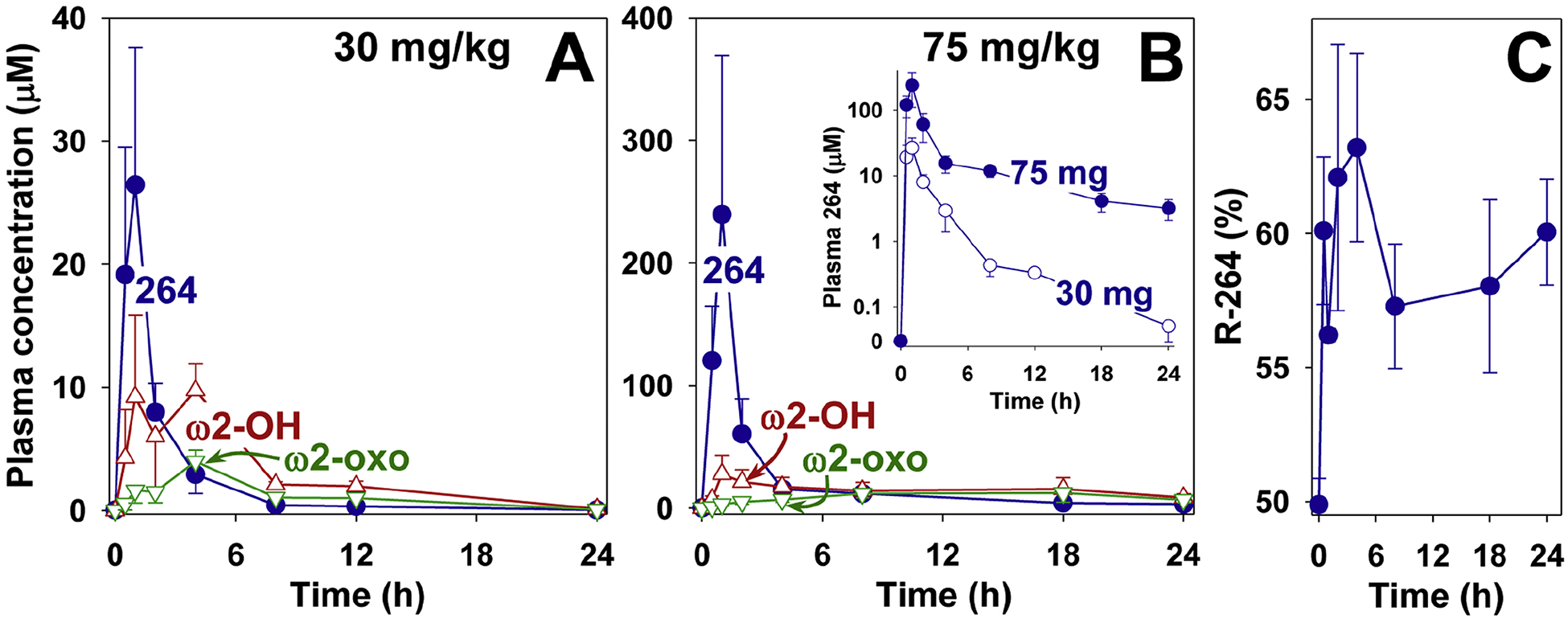
Pharmacokinetics of 264 in monkeys. 264 was administered by oral gavage to cynomolgus monkeys as described in Materials and Methods at doses of either 30 mg/kg (A) or 75 mg/kg (B) and the products were extracted and analyzed by RP-HPLC as shown in the legend to Fig. 5, using 190 as an internal standard. The concentrations of 264 (●), ω2-hydroxy-264 (△), and ω2-oxo-264 (▽) are shown. In the inset to panel B the plasma concentrations of 264 (30 (○) and 75 (●) mg/kg) at different time points are compared using a logarithmic scale. C: The percentage of 264 in the form of the R-enantiomer was determined for each of the time points shown in panel B (264, 75 mg/kg). The S and R enantiomers were separated by chiral HPLC as described in Materials and Methods.
