FIGURE 4.
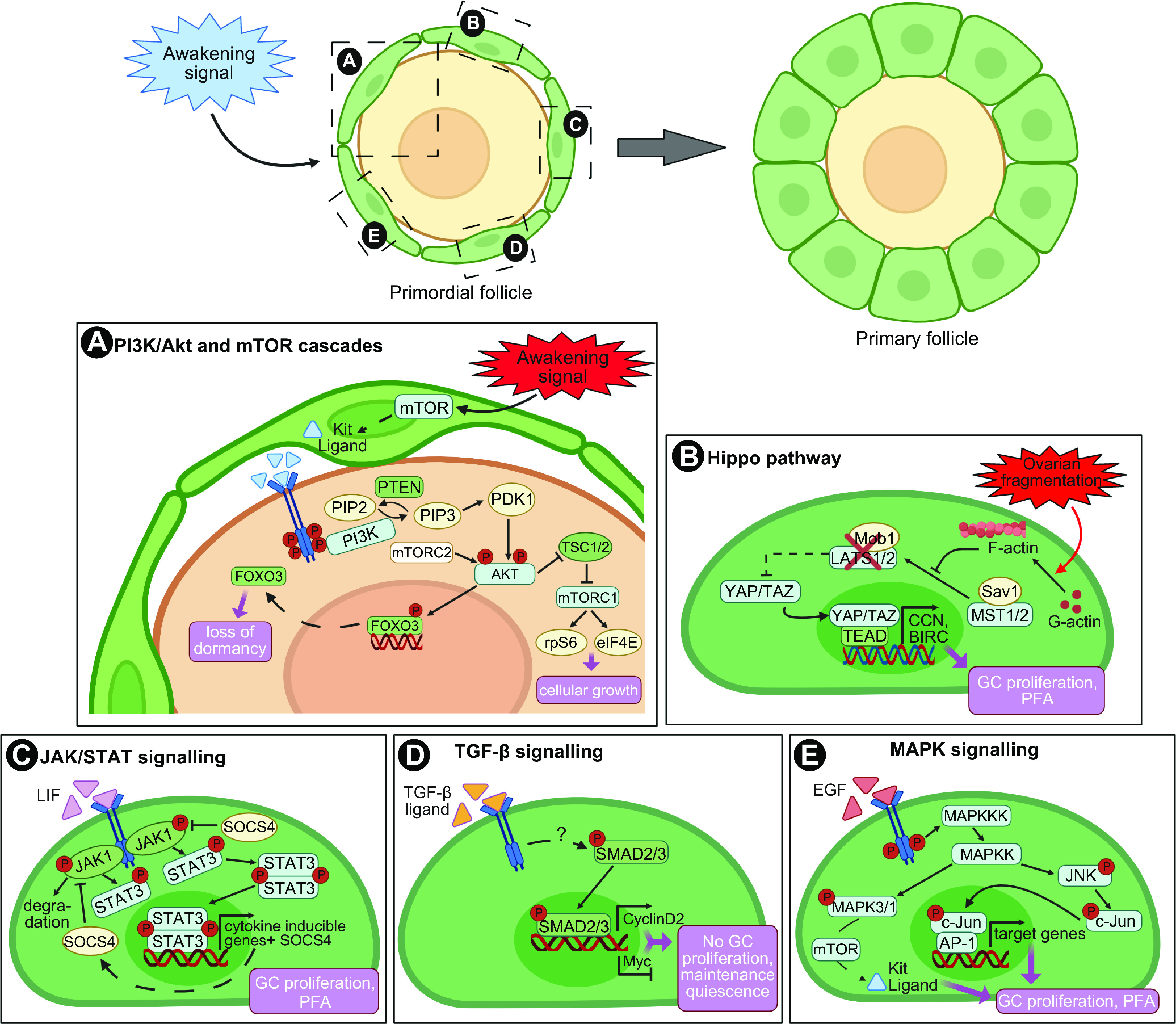
Intrafollicular signaling pathways regulating primordial follicle quiescence and entry into growth. A: granulosa cell (GC) induction of mammalian target of rapamycin (mTOR) leads to the secretion of kit ligand that binds its c-KIT receptor on oocytes, triggering the phosphoinositide-3-kinase (PI3K) cascade. Phosphorylation of AKT triggers nuclear export and suppression of forkhead box O3 (FOXO3) transcription factor activity to promote follicle activation, and induces the activation of the downstream mTOR pathway components to direct cell growth. B: Hippo dysregulation by ovarian fragmentation triggers a switch in the G-actin/F-actin ratio, resulting in the inhibition of LATS1/2 activity and YAP1 dephosphorylation and translocation into the nucleus. YAP/TAZ interaction with TEAD transcription factors promotes the expression of target genes involved in granulosa cell proliferation and primordial follicle activation (PFA). C: activation of the JAK/STAT pathway leads to STAT3 phosphorylation and formation of dimers that translocate to the nucleus, bind to DNA, and regulate transcription of genes involved in GC proliferation and primordial follicle activation. The JAK/STAT activity is negatively regulated by SOCS4. D: GCs from quiescent follicles express the transcription factor SMAD3, which promotes expression of cyclin D2 and represses Myc. Cyclin D2 is bound by the inhibitory factor P27 preventing cell cycle progression while repression of Myc maintains growth arrest. E: activation of the MAPK signaling triggers the phosphorylation of MAPK3/1, which participates in mTOR pathway activation, and JNK, which controls the activity of the proto-oncogene c-Jun and downstream transcription factor AP-1, both promoting GC proliferation and follicle entry into growth. Granulosa cells in green, oocyte in yellow. Image created with BioRender.com, with permission.
