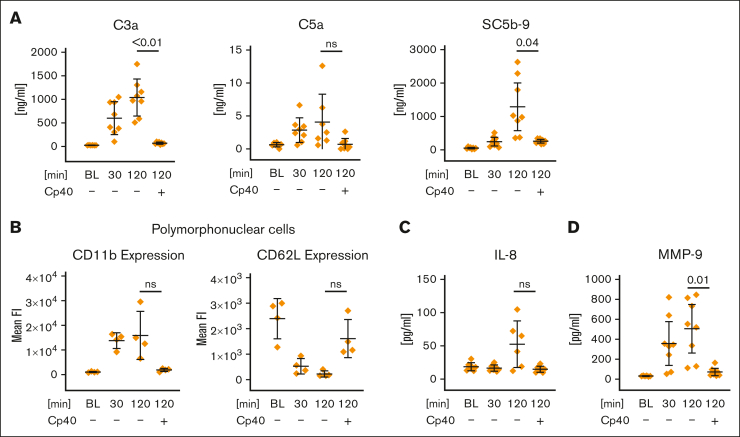
Figure 1.
Artificial surface-induced complement activation and its effect on polymorphonuclear cell activation in whole blood. FPX-anticoagulated blood was incubated for 0 (BL), 30, or 120 minutes at 37°C in the presence or absence of Cp40 (20 μM). (A) Levels of C3a, C5a, and SC5b-9 were determined via enzyme-linked immunosorbent assay (ELISA), using EDTA plasma that was obtained from the whole blood model after each time point. (B) Activation of polymorphonuclear cells. Surface expression of the activation markers CD11b and CD62L was measured via flow cytometry. The average of the mean fluorescence intensities is shown. (C-D) Inflammation marker interleukin-8 (IL-8) and matrix metalloproteinase-9 (MMP-9) were determined from EDTA-treated plasma (derived from the whole blood model) via ELISA. For all panels, the mean values from 4 to 8 independent assays ± standard deviation are shown. Data sets were tested for outliers using the ROUT outlier test (Q = 5%). In the data set of panel A, 2 outliers were removed for each C3a and C5a before statistical testing; in the data set of panel C, 5 and 2 outliers were removed for IL-8 and MMP-9, respectively. Data sets were analyzed using either the Prism mixed-effects model (panels A [C3a, C5a] and C) or repeated measures one-way analysis of variance (ANOVA) (panels A [SC5b-9] and B). Experimental groups were post hoc tested for statistical significance against the 120 minutes + Cp40 group with correction for multiple comparisons (Tukey test, panel A; or Dunnett comparison in panels B-C). For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses. ns, not significant.