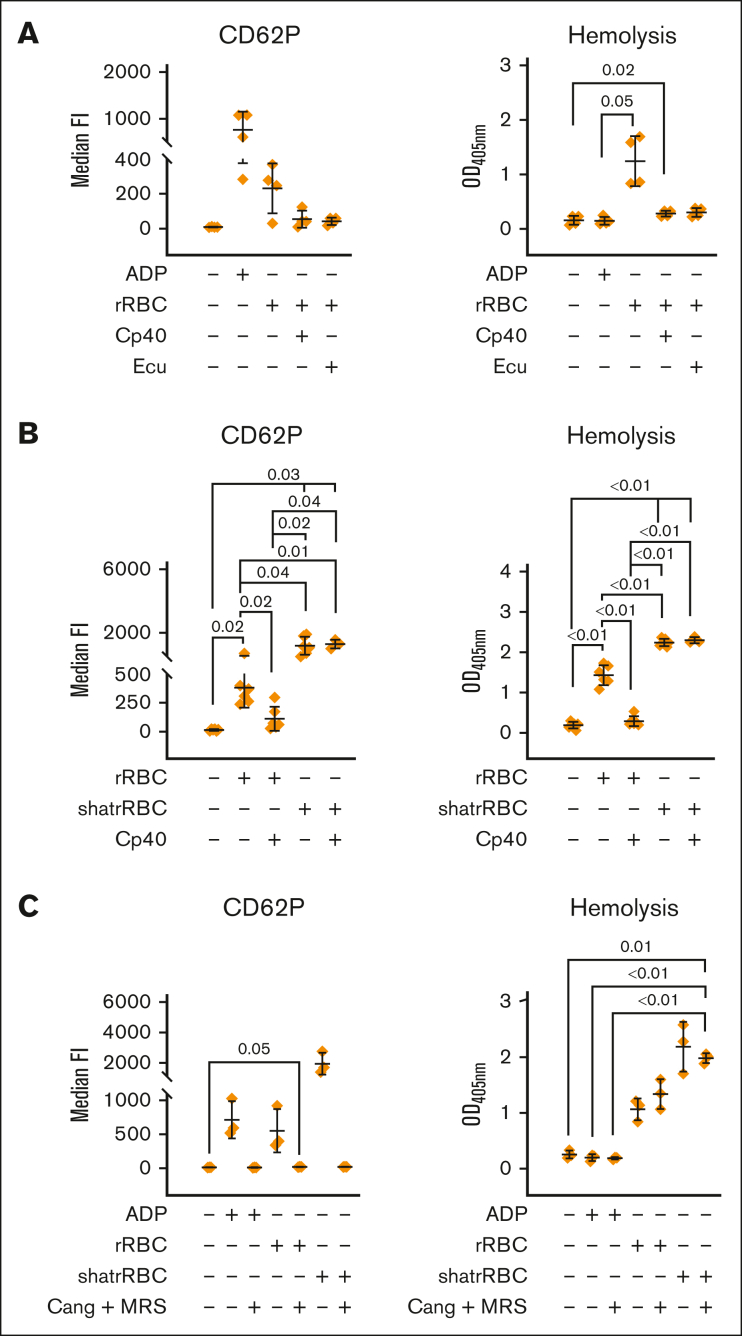
Figure 4.
Complement-dependent and independent lysis of rabbit erythrocytes cause platelet activation, which is abolished via ADP receptor antagonists. (A) Complement-mediated lysis of rRBCs induces platelet activation. Hirudin-anticoagulated blood was exposed to PBS−/− (neg Ctrl), ADP (5 μM), or rRBCs in presence or absence of proximal (Cp40, 4 μM) and terminal (eculizumab [Ecu], 0.4 μM) complement inhibitors. The final blood percentage was 20%. Surface expression of the activation marker CD62P was determined via flow cytometry. Hemolytic activity was determined in respective plasma samples measuring released hemoglobin from the supernatant (after blank subtraction). (B) Complement-dependent and -independent lysis of cells lead to platelet activation. As in panel A, but in addition to intact rRBCs, mechanically shattered rRBCs (shatrRBC) were also assayed with and without complement inhibition by Cp40. All graphs show mean values with standard deviation. (C) Hirudin-anticoagulated blood was exposed to PBS−/− (neg Ctrl), ADP (5 μM), rRBCs, or shatrRBC in presence or absence of ADP receptor antagonists cangrelor (1 μM) or MRS2179 (10 μM). Surface expression of CD62P on platelets was determined by flow cytometry. Hemolytic activity was measured in respective plasma samples. Mean values with standard deviation are shown. Data sets were tested for outliers using the ROUT outlier test (Q = 5%). Data sets were either analyzed using a Prism mixed-effects model (in case of missing values) or repeated measures one-way ANOVA. Experimental groups were post hoc tested for statistical significance with Tukey correction for multiple comparisons. For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses.