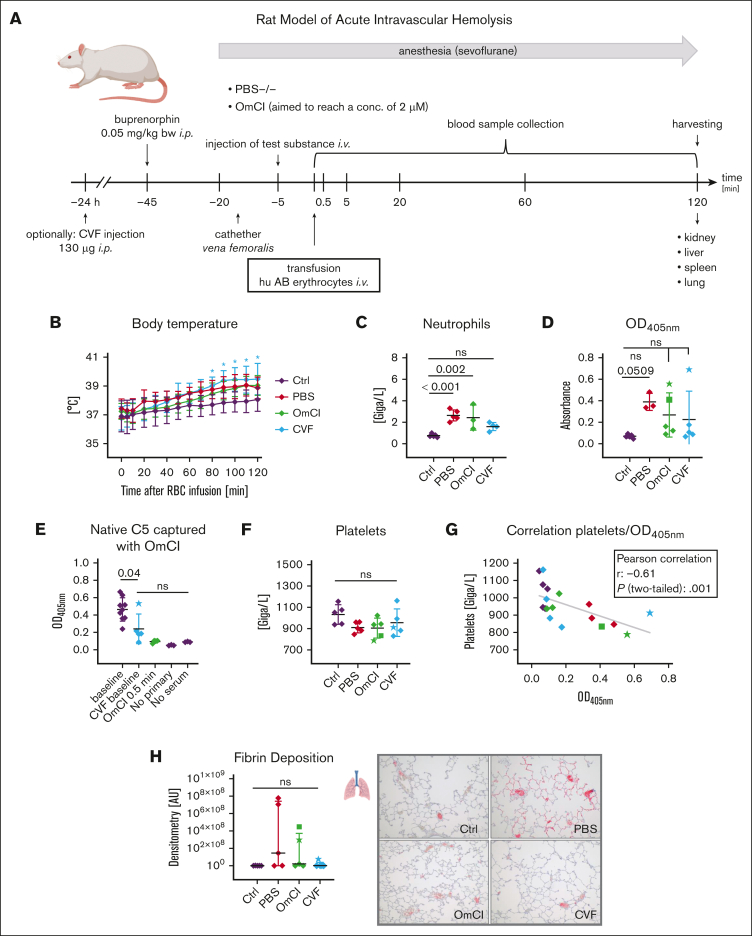
Figure 6.
Rat model of acute intravascular hemolysis. (A) Experimental setup. Human AB erythrocytes were transfused in male Wistar rats simulating a 15% mismatch transfusion to induce an acute intravascular hemolysis. Animals were either pretreated with PBS−/− or OmCI (the aim was a 2-μM final concentration), alternatively, CVF (130 μg) was injected 24 hours before the start of transfusion to allow depletion of the complement system. Blood collection took place at indicated time points after transfusion. After 2 hours, animals were euthanized, and organs were extracted to assess end-organ analysis. (B) Body temperature. Animals were monitored for temperature after transfusion. Mean values with standard deviation are shown at each time point. A star indicates significant difference from control animals without RBC treatment. (C-D) End point clinical blood analysis of neutrophil count and hemolysis levels measured by spectrophotometric analysis of released hemoglobin. Mean values with standard deviation are shown. Star or square symbols in the OmCI and CVF group indicate outlier animals and correspond to star or square values in all other graphs. (E) ELISA-based measurement of nonactivated C5. EDTA-treated plasma samples were added to the surface-coated OmCI-FH8-15 fusion protein, which only binds native, nonactivated C5. Captured C5 was detected using a polyclonal anti-rat C5a antibody (PA5-78891). (F) End point clinical blood analysis of neutrophil count. (G) Correlation between platelets and released hemoglobin (optical density, 405 nm [OD405nm]). (H) Fibrin deposition in lungs was visualized via immunohistochemical staining against fibrinogen β chain and red signal intensity was quantified. From each group, 1 representative image (original magnification ×100) is shown. Data sets were tested for outliers using the ROUT outlier test (Q = 5%). In panel B, each time point was compared with the respective time point in the Ctrl group. Data sets in panels C-F and panel H were analyzed with one-way ANOVA. Experimental groups were post hoc tested for statistical significance against Ctrl/baseline with Dunnett correction for multiple comparisons (panels C-D,F,H) or against each other experimental group (Tukey test with correction for multiple comparisons). In panel G, data sets were tested for correlation using Pearson test. For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses.