Abstract
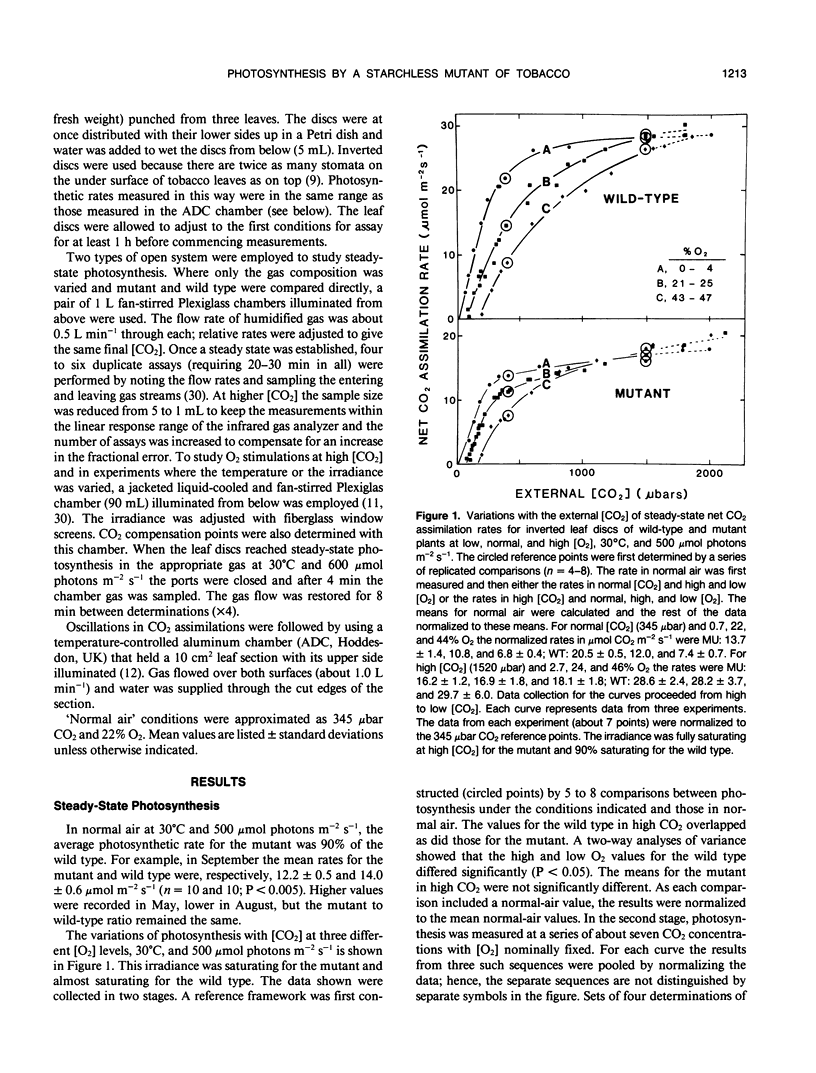
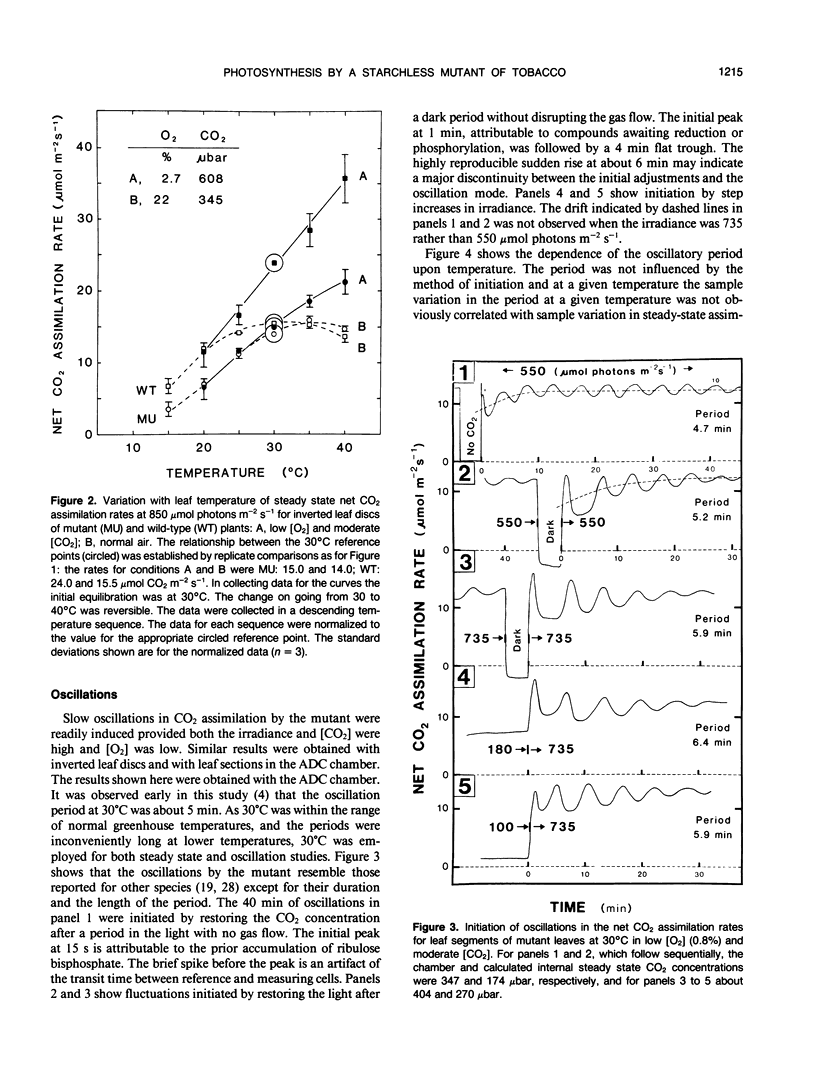
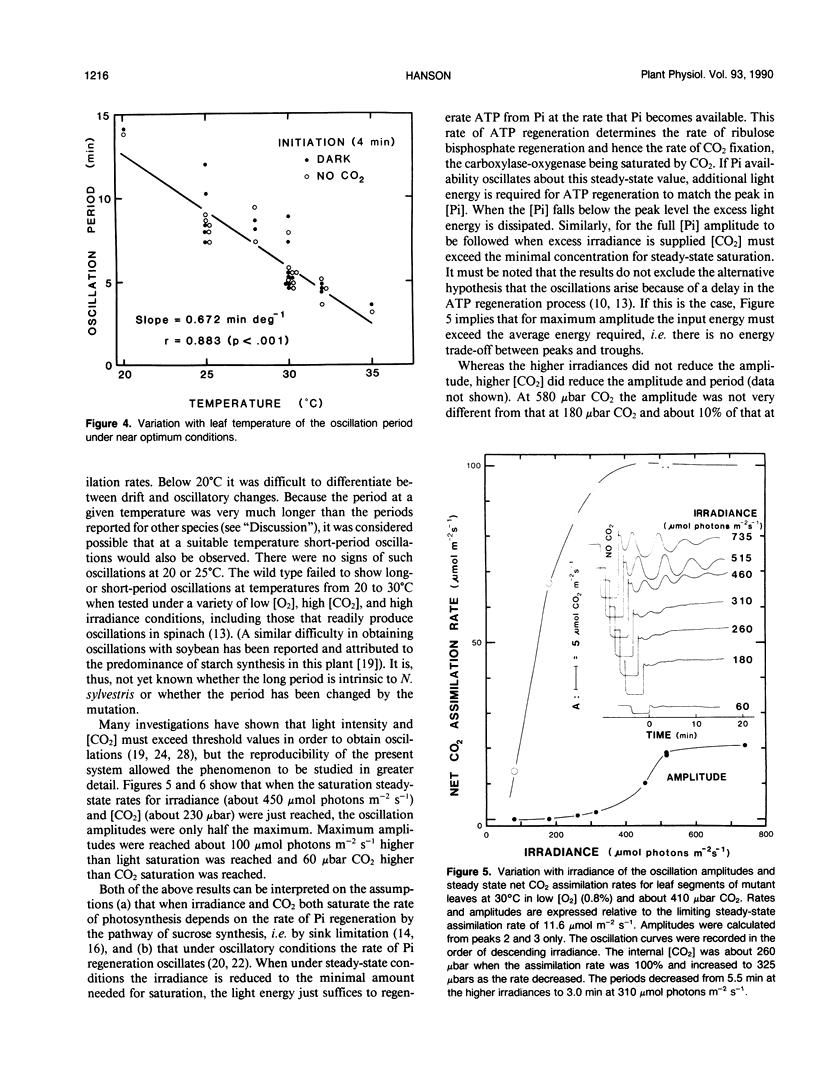
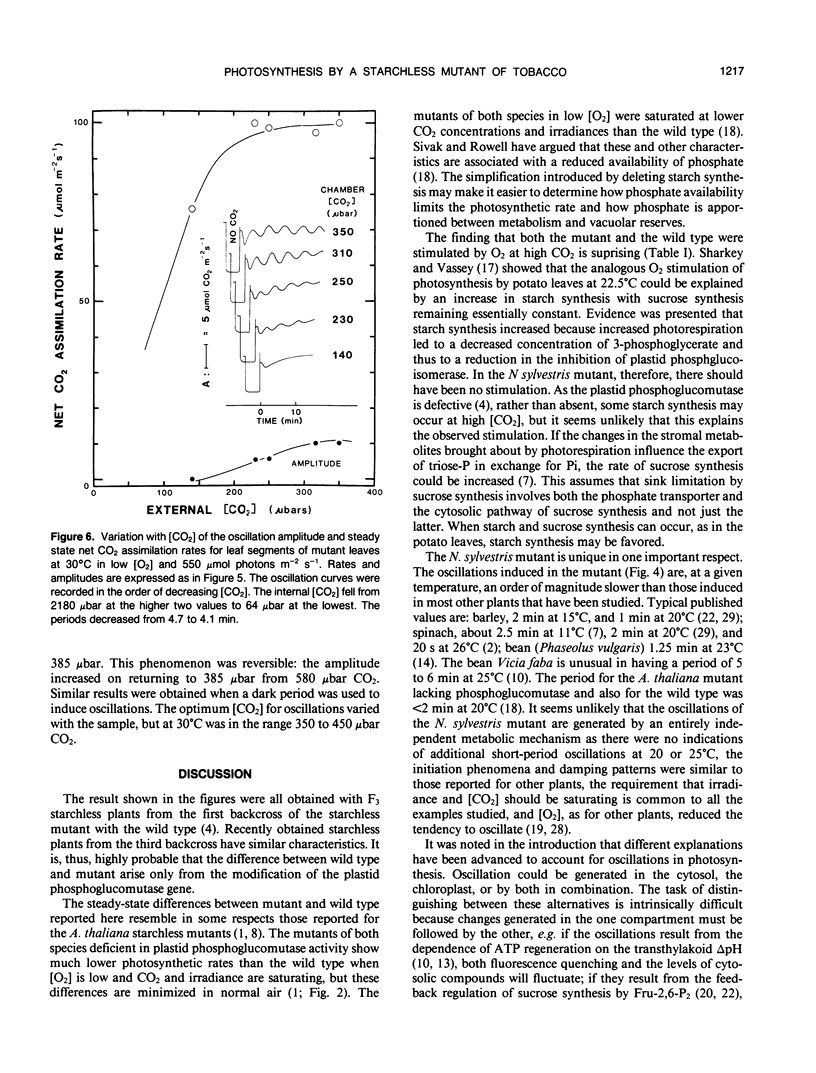
The photosynthetic characteristics of wild type Nicotiana sylvestris (Speg. et Comes) were compared with those of a `starch-less' mutant NS458 that contains a defective plastid phosphoglucomutase (EC 2.1.5.1) (KR Hanson, NA McHale [1988] Plant Physiol 88: 838-844). The steady-state rate of net CO2 assimilation (A) was studied as a function of [CO2], [O2], irradiance, and temperature. At 30°C with saturating light and [CO2] and low [O2], A for the mutant was half that for the wild type, whereas in normal air it was 90%. The irradiance and [CO2] at low [O2] required for saturation were lower than the values for the wild type. At 2000 microbars CO2, 30°C, and saturating irradiance A for both the mutant and wild type was stimulated on going from 4 to 25% O2 by at least 13%. Slow oscillations in A were readily induced with the mutant but not the wild type, provided irradiance and [CO2] were saturating and [O2] was low. The period, which was about 5 minutes at 30°C and decreased by about 0.67 minutes per degree, was an order of magnitude slower than periods reported for other plants at corresponding temperatures. To achieve the full oscillation amplitude both irradiance and [CO2] had to exceed the minimal levels for steady-state saturation. The slowness and duration of the oscillations and the metabolic simplification introduced by deleting starch synthesis makes the mutant especially suitable for investigating the regulatory processes that generate such oscillations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspar T., Huber S. C., Somerville C. Alterations in Growth, Photosynthesis, and Respiration in a Starchless Mutant of Arabidopsis thaliana (L.) Deficient in Chloroplast Phosphoglucomutase Activity. Plant Physiol. 1985 Sep;79(1):11–17. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank R. T., Foyer C. H. Oscillations in levels of metabolites from the photosynthetic carbon reduction cycle in spinach leaf disks generated by the transition from air to 5% CO2. Arch Biochem Biophys. 1986 Apr;246(1):240–244. doi: 10.1016/0003-9861(86)90469-8. [DOI] [PubMed] [Google Scholar]
- Hanson K. R., McHale N. A. A Starchless Mutant of Nicotiana sylvestris Containing a Modified Plastid Phosphoglucomutase. Plant Physiol. 1988 Nov;88(3):838–844. doi: 10.1104/pp.88.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. C., Cheesbrough J. K., Walker D. A. Measurement of CO(2) and H(2)O Vapor Exchange in Spinach Leaf Discs : Effects of Orthophosphate. Plant Physiol. 1983 Jan;71(1):102–107. doi: 10.1104/pp.71.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C., Preiss J. Isolation and Characterization of a Starchless Mutant of Arabidopsis thaliana (L.) Heynh Lacking ADPglucose Pyrophosphorylase Activity. Plant Physiol. 1988 Apr;86(4):1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. Estimation of Photorespiration Based on the Initial Rate of Postillumination CO(2) Release: II. Effects of O(2), CO(2), and Temperature. Plant Physiol. 1983 Dec;73(4):983–988. doi: 10.1104/pp.73.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. Partitioning of Noncyclic Photosynthetic Electron Transport to O(2)-Dependent Dissipative Processes as Probed by Fluorescence and CO(2) Exchange. Plant Physiol. 1989 Aug;90(4):1322–1328. doi: 10.1104/pp.90.4.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Sivak M. N., Walker D. A. Carbon Dioxide-Induced Oscillations in Fluorescence and Photosynthesis: Role of Thylakoid Membrane Energization in Regulation of Photosystem II Activity. Plant Physiol. 1988 Dec;88(4):1125–1130. doi: 10.1104/pp.88.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Drake R. R., Broadbent K. P., Haley B. E., Hanson K. R., McHale N. A. Identification of the 64 kilodalton chloroplast stromal phosphoprotein as phosphoglucomutase. Plant Physiol. 1990 May;93(1):105–109. doi: 10.1104/pp.93.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Seemann J. R., Berry J. A. Regulation of Ribulose-1,5-Bisphosphate Carboxylase Activity in Response to Changing Partial Pressure of O(2) and Light in Phaseolus vulgaris. Plant Physiol. 1986 Jul;81(3):788–791. doi: 10.1104/pp.81.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Vassey T. L. Low oxygen inhibition of photosynthesis is caused by inhibition of starch synthesis. Plant Physiol. 1989 Jun;90(2):385–387. doi: 10.1104/pp.90.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Sivak M. N., Prinsley R. T., Cheesbrough J. K. Simultaneous measurement of oscillations in oxygen evolution and chlorophyll a fluorescence in leaf pieces. Plant Physiol. 1983 Nov;73(3):542–549. doi: 10.1104/pp.73.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Selection and characterization of tobacco plants with novel o(2)-resistant photosynthesis. Plant Physiol. 1989 Aug;90(4):1457–1464. doi: 10.1104/pp.90.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]


