Abstract
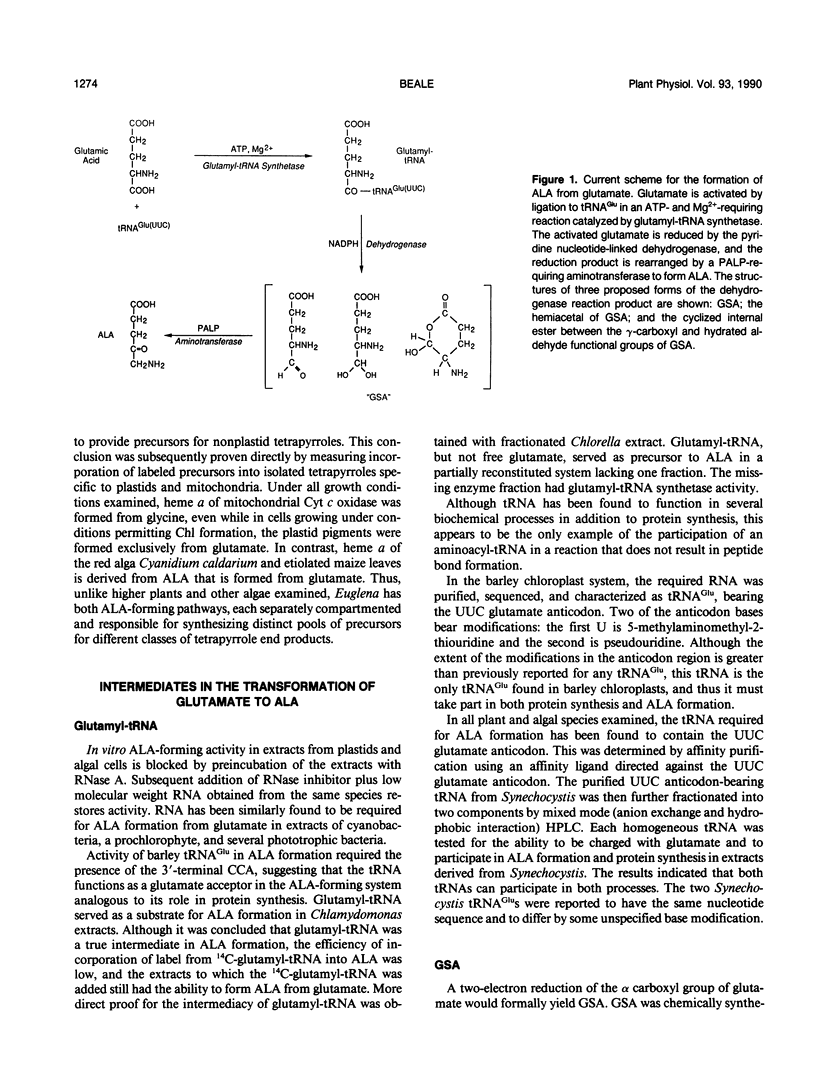
δ-Aminolevulinic acid (ALA), the common biosynthetic precursor of hemes, chlorophylls, and bilins, is synthesized by two distinct routes. Among phototrophic species, purple nonsulfur bacteria form ALA by condensation of glycine with succinyl-CoA, catalyzed by ALA synthase, in a reaction identical to that occurring in the mitochondria of animals, yeast, and fungi. Most or all other phototrophic species form ALA exclusively from the intact carbon skeleton of glutamic acid in a reaction sequence that begins with activation of the α-carboxyl group of glutamate by an ATP-dependent ligation to tRNAGlu, catalyzed by glutamyl-tRNA synthetase. Glutamyl-tRNA is the substrate for a pyridine nucleotide-dependent dehydrogenase reaction whose product is glutamate-1-semialdehyde or a similar reduced compound. Glutamate-1-semialdehyde is then transaminated to form ALA. Regulation of ALA formation from glutamate is exerted at the dehydrogenase step through end product feedback inhibition and induction/repression. In some species, end product inhibition of the glutamyl-tRNA synthetase step and developmental regulation of tRNAGlu level may also occur.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Beale S. I. Biosynthesis of Tetrapyrrole Pigment Precursors : Pyridoxal Requirement of the Aminotransferase Step in the Formation of delta-Aminolevulinate from Glutamate in Extracts of Chlorella vulgaris. Plant Physiol. 1989 Mar;89(3):852–859. doi: 10.1104/pp.89.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Cloning and expression of a structural gene from Chlorobium vibrioforme that complements the hemA mutation in Escherichia coli. J Bacteriol. 1990 Mar;172(3):1656–1659. doi: 10.1128/jb.172.3.1656-1659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J Bacteriol. 1989 Jun;171(6):2919–2924. doi: 10.1128/jb.171.6.2919-2924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Ormerod J. G., Beale S. I. Distribution of delta-aminolevulinic acid biosynthetic pathways among phototrophic bacterial groups. Arch Microbiol. 1989;151(6):513–519. doi: 10.1007/BF00454867. [DOI] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm B., Bull A., Welinder K. G., Gough S. P., Kannangara C. G. Purification and partial amino acid sequence of the glutamate 1-semialdehyde aminotransferase of barley and synechococcus. Carlsberg Res Commun. 1989;54(2):67–79. doi: 10.1007/BF02907586. [DOI] [PubMed] [Google Scholar]
- Huang L., Bonner B. A., Castelfranco P. A. Regulation of 5-Aminolevulinic Acid (ALA) Synthesis in Developing Chloroplasts : II. Regulation of ALA-Synthesizing Capacity by Phytochrome. Plant Physiol. 1989 Jul;90(3):1003–1008. doi: 10.1104/pp.90.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Castelfranco P. A. Regulation of 5-aminolevulinic Acid synthesis in developing chloroplasts : I. Effect of light/dark treatments in vivo and in organello. Plant Physiol. 1989 Jul;90(3):996–1002. doi: 10.1104/pp.90.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. M., Brathwaite O., Cosloy S. D., Russell C. S. 5-Aminolevulinic acid synthesis in Escherichia coli. J Bacteriol. 1989 May;171(5):2547–2552. doi: 10.1128/jb.171.5.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G. P., Chen M. W., Söll D. delta-Aminolevulinic acid biosynthesis in Escherichia coli and Bacillus subtilis involves formation of glutamyl-tRNA. FEMS Microbiol Lett. 1989 Aug;51(3):255–259. doi: 10.1016/0378-1097(89)90406-0. [DOI] [PubMed] [Google Scholar]
- Rieble S., Ormerod J. G., Beale S. I. Transformation of glutamate to delta-aminolevulinic acid by soluble extracts of Chlorobium vibrioforme. J Bacteriol. 1989 Jul;171(7):3782–3787. doi: 10.1128/jb.171.7.3782-3787.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


