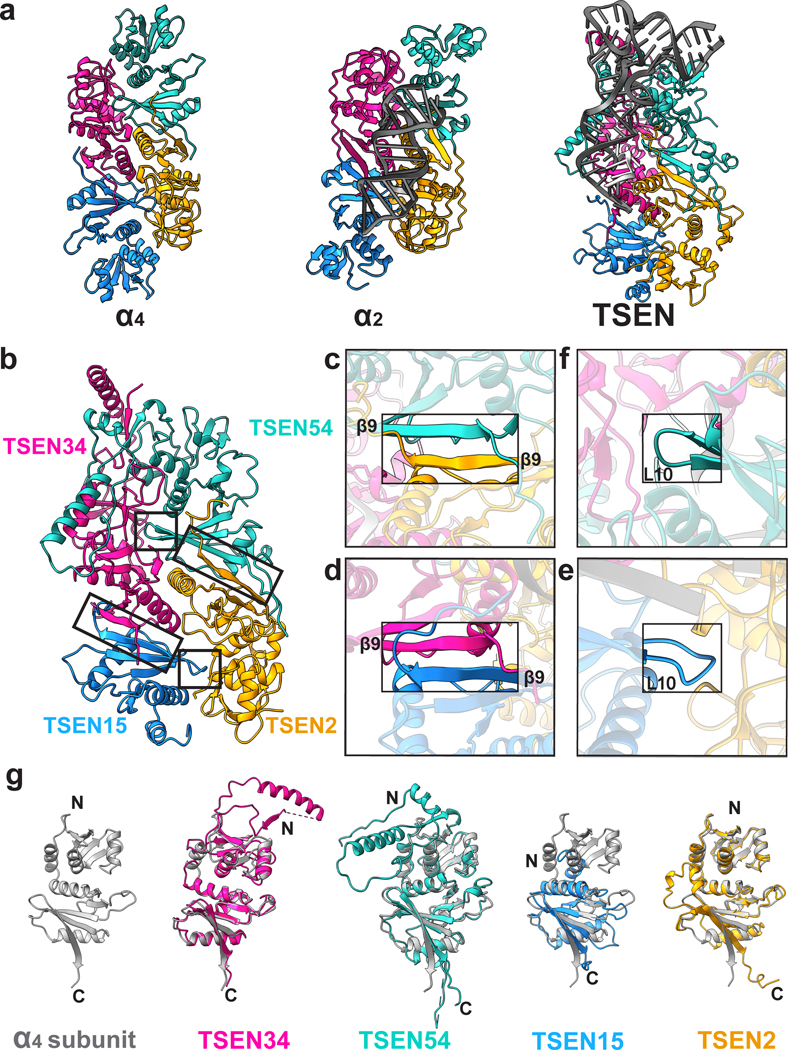
Fig. 2|. The human TSEN complex retains the core architecture from the archaeal complex.
a. Structures of a homotetrameric (α4, PDBID: 1A79), homodimeric BHB bound (α2, PDBID:2GJW), and the pre-tRNA bound TSEN complex, colored to mimic the arrangement of the subunits within the TSEN complex (TSEN54 – teal, endonuclease TSEN2 - orange, endonuclease TSEN34 – pink, TSEN15 – medium blue). b. The TSEN complex retains β9-β9 interactions at the C-terminal domain of endonuclease/structural protein interfaces: c. TSEN2:TSEN54 and d. TSEN34:TSEN15. The L10-loop of the structural proteins e. TSEN54 and f. TSEN15 link the β9-β9 heterodimers together. g. A single archaeal homotetramer α subunit (PDBID: 1A79, grey) superimposed with each individual TSEN subunit.