Figure 4: CRL3s controlling neuroectodermal development.
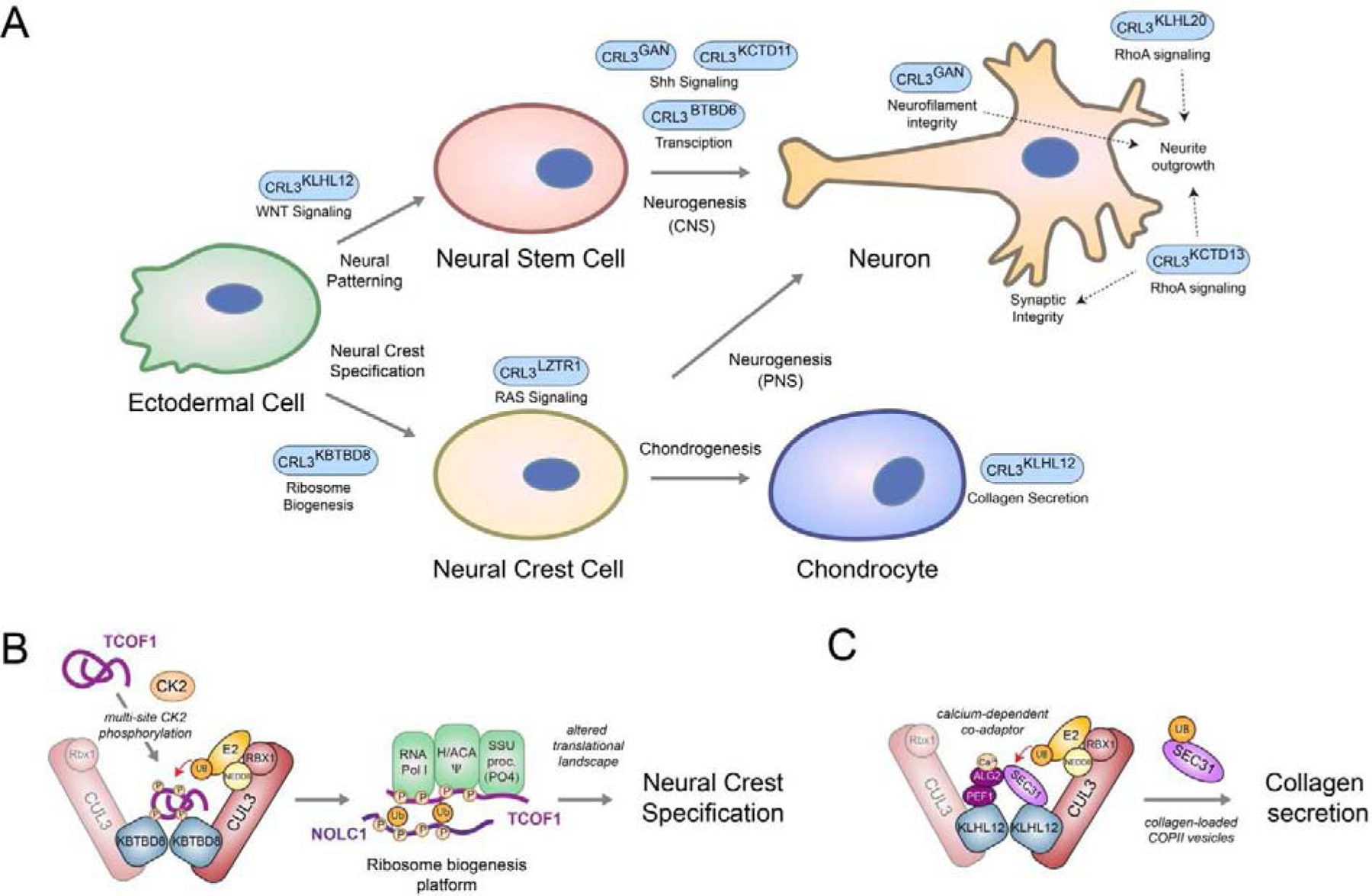
A) Schematic overview of various CRL3 complexes implicated in regulating aspects of neuroectodermal differentiation. B) Model of CRL3-KBTBD8-dependent neural crest specification. During embryonic development, multisite CK2-phopshorylation of the ribosome biogenesis factors TCOF1 and NOLC1 allows their recognition and monoubiquitylation by homo-dimeric CRL3-KBTBD8. This is thought to open the compact conformation of these ampholytic, intrinsically disordered proteins to promote recognition of ribosome biogenesis factors, including the RNA polymerase I, the pseudouridylation machinery (Ψ), and the SSU processome. These ubiquitin-dependent changes in ribosome biogenesis then trigger remodeling of translational networks required for neural crest specification, which likely occurs through newly synthesized, modified ribosomes C) Model of calcium-induced CRL3-KLHL12-dependent collagen secretion. During bone formation, homo-dimeric CRL3-KLHL12 complexes are thought to engage their substrate SEC31 using a co-adaptor complex consisting of PEF1 and ALG2. The ALG2 subunit is only able to engage SEC31 after calcium has been released from the endoplasmic reticulum, thus allowing CRL3-KLHL12 to mono-ubiquitylate and promote formation of large, collagen-loaded COPII vesicles in a calcium-dependent manner.
